Abstract
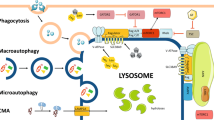
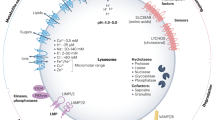
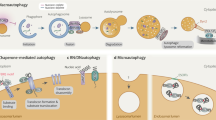
Exciting new discoveries have transformed the view of the lysosome from a static organelle dedicated to the disposal and recycling of cellular waste to a highly dynamic structure that mediates the adaptation of cell metabolism to environmental cues. Lysosome-mediated signalling pathways and transcription programmes are able to sense the status of cellular metabolism and control the switch between anabolism and catabolism by regulating lysosomal biogenesis and autophagy. The lysosome also extensively communicates with other cellular structures by exchanging content and information and by establishing membrane contact sites. It is now clear that lysosome positioning is a dynamically regulated process and a crucial determinant of lysosomal function. Finally, growing evidence indicates that the role of lysosomal dysfunction in human diseases goes beyond rare inherited diseases, such as lysosomal storage disorders, to include common neurodegenerative and metabolic diseases, as well as cancer. Together, these discoveries highlight the lysosome as a regulatory hub for cellular and organismal homeostasis, and an attractive therapeutic target for a broad variety of disease conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
De Duve, C., Pressman, B. C., R, G. I., Wattieaux, R. & Appelmans, F. Tissue fractionation studies. 6. intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 60, 604–617 (1955).
Saftig, P. & Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009).
Hesketh, G. G., Wartosch, L., Davis, L. J., Bright, N. A. & Luzio, J. P. The lysosome and intracellular signalling. Prog. Mol. Subcell. Biol. 57, 151–180 (2018).
Tait, S. W. & Green, D. R. Mitochondria and cell signalling. J. Cell Sci. 125, 807–815 (2012).
Schiaffino, M. V. et al. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat. Genet. 23, 108–112 (1999).
Tripathi, D. N. & Walker, C. L. The peroxisome as a cell signaling organelle. Curr. Opin. Cell Biol. 39, 109–112 (2016).
Sancak, Y. et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010). This landmark article demonstrates that mTORC1 exerts its activity on the lysosomal surface to which it is recruited by the nutrient-activated RAG–Ragulator complex.
Settembre, C., Fraldi, A., Medina, D. L. & Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017).
Hosokawa, N. et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991 (2009).
Yu, L. et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465, 942–946 (2010).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Kim, E., Goraksha-Hicks, P., Li, L., Neufeld, T. P. & Guan, K. L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10, 935–945 (2008).
de Araujo, M. E. G. et al. Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science 358, 377–381 (2017).
Lawrence, R. E. et al. A nutrient-induced affinity switch controls mTORC1 activation by its Rag GTPase-Ragulator lysosomal scaffold. Nat. Cell Biol. 20, 1052–1063 (2018).
Su, M. Y. et al. Hybrid structure of the RagA/C-Ragulator mTORC1 activation complex. Mol. Cell 68, 835–846.e3 (2017).
Lawrence, R. E. & Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133–142 (2019).
Castellano, B. M. et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 355, 1306–1311 (2017).
Demetriades, C., Doumpas, N. & Teleman, A. A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 156, 786–799 (2014).
Petit, C. S., Roczniak-Ferguson, A. & Ferguson, S. M. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J. Cell Biol. 202, 1107–1122 (2013).
Martina, J. A. & Puertollano, R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J. Cell Biol. 200, 475–491 (2013).
Morgan, A. J., Platt, F. M., Lloyd-Evans, E. & Galione, A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 439, 349–374 (2011).
Li, P., Gu, M. & Xu, H. Lysosomal ion channels as decoders of cellular signals. Trends Biochem. Sci. 44, 110–124 (2019).
Wang, W. et al. A voltage-dependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores. J. Cell Biol. 216, 1715–1730 (2017).
Mindell, J. A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86 (2012).
Cheng, X., Shen, D., Samie, M. & Xu, H. Mucolipins: intracellular TRPML1-3 channels. FEBS Lett. 584, 2013–2021 (2010).
Bassi, M. T. et al. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J. Hum. Genet. 67, 1110–1120 (2000).
Bargal, R. et al. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 26, 118–123 (2000).
Medina, D. L. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015).
Wang, W. et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl Acad. Sci. USA 112, E1373–E1381 (2015).
Zhang, X. et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 7, 12109 (2016).
Dong, X. P. et al. PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 1, 38 (2010).
Reddy, A., Caler, E. V. & Andrews, N. W. Plasma membrane repair is mediated by Ca2+ regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Cao, Q., Yang, Y., Zhong, X. Z. & Dong, X. P. The lysosomal Ca2+ release channel TRPML1 regulates lysosome size by activating calmodulin. J. Biol. Chem. 292, 8424–8435 (2017).
Miller, A. et al. Mucolipidosis type IV protein TRPML1-dependent lysosome formation. Traffic 16, 284–297 (2015).
Medina, D. L. et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430 (2011).
Samie, M. et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell 26, 511–524 (2013).
Bretou, M. et al. Lysosome signaling controls the migration of dendritic cells. Sci Immunol. 2, eaak9573 (2017).
Aits, S. & Jäättelä, M. Lysosomal cell death at a glance. J. Cell Sci. 126, 1905–1912 (2013).
Repnik, U., Stoka, V., Turk, V. & Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 1824, 22–33 (2012).
Wang, F., Gómez-Sintes, R. & Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 19, 918–931 (2018).
Shi, J., Gao, W. & Shao, F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 (2017).
Cao, J. Y. & Dixon, S. J. Mechanisms of ferroptosis. Cell Mol. Life Sci. 73, 2195–2209 (2016).
Vanden Berghe, T. et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 (2010).
Papadopoulos, C. & Meyer, H. Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Curr. Biol. 27, R1330–R1341 (2017).
Maejima, I. et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347 (2013).
Chauhan, S. et al. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev. Cell 39, 13–27 (2016).
Reymond, A. et al. The tripartite motif family identifies cell compartments. EMBO J. 20, 2140–2151 (2001).
Thurston, T. L., Wandel, M. P., von Muhlinen, N., Foeglein, A. & Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–418 (2012).
Jia, J. et al. Galectins control mTOR in response to endomembrane damage. Mol. Cell 70, 120–135 (2018).
Radulovic, M. et al. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 37, e99753 (2018).
Skowyra, M. L., Schlesinger, P. H., Naismith, T. V. & Hanson, P. I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360, eaar5078 (2018). This study elegantly shows that the ESCRT machinery is recruited to injured endolysosomes to allow their recovery from damage.
Matz, K. M., Guzman, R. M. & Goodman, A. G. The role of nucleic acid sensing in controlling microbial and autoimmune disorders. Int. Rev. Cell Mol. Biol. 345, 35–136 (2019).
Vidya, M. K. et al. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 37, 20–36 (2018).
Majer, O., Liu, B. & Barton, G. M. Nucleic acid-sensing TLRs: trafficking and regulation. Curr. Opin. Immunol. 44, 26–33 (2017).
De Leo, M. G. et al. Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat. Cell Biol. 18, 839–850 (2016). This article describes the discovery of a TRLR9–OCRL–TRPML1-mediated mechanism that allows the lysosome to respond to the arrival of autophagic cargo.
Thelen, A. M. & Zoncu, R. Emerging roles for the lysosome in lipid metabolism. Trends Cell Biol. 27, 833–850 (2017).
Ebner, M., Koch, P. A. & Haucke, V. Phosphoinositides in the control of lysosome function and homeostasis. Biochem. Soc. Trans. 47, 1173–1185 (2019).
Folick, A. et al. Ageing. lysosomal signalling molecules regulate longevity in Caenorhabditis elegans. Science 347, 83–86 (2015).
Ramachandran, P. V. et al. Lysosomal signalling promotes longevity by adjusting mitochondrial activity. Dev. Cell 48, 685–696.e5 (2019).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011). Sardiello et al. (2009) and Settembre et al. (2011) describe the discovery of a lysosomal–autophagic gene network and its master regulator TFEB, the first example of global transcriptional control of lysosomal function.
Hemesath, T. J. et al. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 8, 2770–2780 (1994).
Palmieri, M. et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 (2011).
Willett, R. et al. TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes. Nat. Commun. 8, 1580 (2017).
Martina, J. A. et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal 7, ra9 (2014).
Pastore, N. et al. TFE3 regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol. Med. 9, 605–621 (2017).
Rega, L. R. et al. Activation of the transcription factor EB rescues lysosomal abnormalities in cystinotic kidney cells. Kidney Int. 89, 862–873 (2016).
Spampanato, C. et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol. Med. 5, 691–706 (2013).
Chauhan, S. et al. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat. Commun. 6, 8620 (2015).
Decressac, M. et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl Acad. Sci. USA 110, E1817–E1826 (2013).
Polito, V. A. et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol. Med. 6, 1142–1160 (2014).
Xiao, Q. et al. Neuronal-targeted TFEB accelerates lysosomal degradation of APP, reducing Aβ generation and amyloid plaque pathogenesis. J. Neurosci. 35, 12137–12151 (2015).
Pastore, N. et al. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol. Med. 5, 397–412 (2013).
Settembre, C. et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 (2013).
Napolitano, G. & Ballabio, A. TFEB at a glance. J. Cell Sci. 129, 2475–2481 (2016).
Martina, J. A., Diab, H. I., Brady, O. A. & Puertollano, R. TFEB and TFE3 are novel components of the integrated stress response. EMBO J. 35, 479–495 (2016).
Nnah, I. C. et al. TFEB-driven endocytosis coordinates MTORC1 signalling and autophagy. Autophagy. 15, 151–164 (2019).
Betschinger, J. et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell 153, 335–347 (2013).
Leeman, D. S. et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during ageing. Science 359, 1277–1283 (2018).
Villegas, F. et al. Lysosomal signalling licenses embryonic stem cell differentiation via inactivation of Tfe3. Cell Stem Cell 24, 257–270.e8 (2019).
Curnock, R., Calcagni, A., Ballabio, A. B. & Cullen, P. J. TFEB controls retromer expression in response to nutrient availability. J. Cell Biol. https://doi.org/10.1083/jcb.201903006 (2019).
Pastore, N. et al. Nutrient-sensitive transcription factors TFEB and TFE3 couple autophagy and metabolism to the peripheral clock. EMBO J. 38, e101347 (2019).
Visvikis, O. et al. Innate host defence requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity 40, 896–909 (2014).
Grey, M. A. et al. Phagocytosis enhances lysosomal and bactericidal properties by activating the transcription factor TFEB. Curr. Biol. 26, 1955–1964 (2016).
Pastore, N. et al. TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy 12, 1240–1258 (2016).
Mansueto, G. et al. Transcription factor eb controls metabolic flexibility during exercise. Cell Metab. 25, 182–196 (2017).
Martina, J. A. & Puertollano, R. Protein phosphatase 2A stimulates activation of TFEB and TFE3 transcription factors in response to oxidative stress. J. Biol. Chem. 293, 12525–12534 (2018).
Nezich, C. L., Wang, C., Fogel, A. I. & Youle, R. J. MiT/TFE transcription factors are activated during mitophagy downstream of parkin and Atg5. J. Cell Biol. 210, 435–450 (2015).
Puertollano, R., Ferguson, S. M., Brugarolas, J. & Ballabio, A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J. 37, e98804 (2018).
Peña-Llopis, S. et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 30, 3242–3258 (2011).
Martina, J. A., Chen, Y., Gucek, M. & Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012).
Roczniak-Ferguson, A. et al. The transcription factor TFEB links mTORC1 signalling to transcriptional control of lysosome homeostasis. Sci. Signal 5, ra42 (2012).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012). Peña-Llopis et al. (2001), Martina et al. (2012), Roczniak-Ferguson et al. (2012) and Settembre et al. (2012) demonstrate that the nutrient-regulated mTORC1 kinase complex phosphorylates TFEB and controls its nuclear translocation. This mechanism allows lysosomal function to respond to environmental cues such a nutrient availability.
Vega-Rubin-de-Celis, S., Peña-Llopis, S., Konda, M. & Brugarolas, J. Multistep regulation of TFEB by MTORC1. Autophagy 13, 464–472 (2017).
Di Malta, C. et al. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 356, 1188–1192 (2017).
Napolitano, G. et al. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat Commun. 9, 3312 (2018).
Li, L. et al. A TFEB nuclear export signal integrates amino acid supply and glucose availability. Nat. Commun. 9, 2685 (2018).
Silvestrini, M. J. et al. Nuclear export inhibition enhances HLH-30/TFEB activity, autophagy, and lifespan. Cell Rep. 23, 1915–1921 (2018).
Sha, Y., Rao, L., Settembre, C., Ballabio, A. & Eissa, N. T. STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J. 36, 2544–2552 (2017).
Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 30621, S1097–S2765 (2019).
Sakamaki, J. I. et al. Bromodomain protein BRD4 is a transcriptional repressor of autophagy and lysosomal function. Mol. Cell 66, 517–532.e9 (2017).
Kreuzaler, P. A. et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat. Cell Biol. 13, 303–309 (2011).
Martínez-Fábregas, J. et al. Lysosomal protease deficiency or substrate overload induces an oxidative-stress mediated STAT3-dependent pathway of lysosomal homeostasis. Nat Commun. 9, 5343 (2018).
Liu, B. et al. STAT3 associates with vacuolar H+-ATPase and regulates cytosolic and lysosomal pH. Cell Res. 28, 996–1012 (2018).
Shin, H. J. et al. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 534, 553–557 (2016).
Chauhan, S. et al. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol. Cell. 50, 16–28 (2013).
Annunziata, I. et al. MYC competes with MiT/TFE in regulating lysosomal biogenesis and autophagy through an epigenetic rheostat. Nat. Commun. 10, 3623 (2019).
Li, Y. et al. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat. Cell Biol. 18, 1065–1077 (2016).
Garg, S. et al. Lysosomal trafficking, antigen presentation, and microbial killing are controlled by the Arf-like GTPase Arl8b. Immunity 35, 182–193 (2011).
McEwan, D. G. et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell 57, 39–54 (2015).
Marwaha, R. et al. The Rab7 effector PLEKHM1 binds Arl8b to promote cargo traffic to lysosomes. J. Cell Biol. 216, 1051–1070 (2017).
Antonin, W. et al. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 19, 6453–6464 (2000).
Zhao, Y. G. & Zhang, H. Autophagosome maturation: an epic journey from the ER to lysosomes. J. Cell Biol. 218, 757–770 (2019).
Jia, R., Guardia, C. M., Pu, J., Chen, Y. & Bonifacino, J. S. BORC coordinates encounter and fusion of lysosomes with autophagosomes. Autophagy 13, 1648–1663 (2017).
Wang, Z. et al. The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol. Cell 63, 781–795 (2016).
Tabata, K. et al. Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol. Biol. Cell 21, 4162–4172 (2010).
Kim, Y. M. et al. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol. Cell 57, 207–218 (2015).
Naegeli, K. M. et al. Cell invasion in vivo via rapid exocytosis of a transient lysosome-derived membrane domain. Dev. Cell 43, 403–417.e10 (2017). This study uncovers a mechanism by which lysosome exocytosis helps form an invasive protrusion that breaches tissue barriers during C. elegans development, a process that may be analogous to cancer cell invasion.
Baron, R. et al. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J. Cell Biol. 106, 1863–1872 (1988).
Saffi, G. T. & Botelho, R. J. Lysosome fission: planning for an exit. Trends. Cell Biol. 29, 635–646 (2019).
Levin-Konigsberg, R. et al. Phagolysosome resolution requires contacts with the endoplasmic reticulum and phosphatidylinositol-4-phosphate signalling. Nat. Cell Biol. 21, 1234–1247 (2019).
Krajcovic, M., Krishna, S., Akkari, L., Joyce, J. A. & Overholtzer, M. mTOR regulates phagosome and entotic vacuole fission. Mol. Biol. Cell 24, 3736–3745 (2013).
Wu, H., Carvalho, P. & Voeltz, G. K. Here, there, and everywhere: the importance of ER membrane contact sites. Science 361, eaan5835 (2018).
Friedman, J. R., Dibenedetto, J. R., West, M., Rowland, A. A. & Voeltz, G. K. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 24, 1030–1040 (2013).
Dong, R. et al. Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell 166, 408–423 (2016).
Luo, J., Jiang, L., Yang, H. & Song, B. L. Routes and mechanisms of post-endosomal cholesterol trafficking: a story that never ends. Traffic 18, 209–217 (2017).
Wilhelm, L. P. et al. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 36, 1412–1433 (2017).
Kumar, N. et al. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 217, 3625–3639 (2018). This article reports that the protein VPS13C, which is mutated in some forms of Parkinson disease, is a lipid transport protein that tethers late endosomes/lysosomes to the ER.
Rocha, N. et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150Glued and late endosome positioning. J. Cell Biol. 185, 1209–1225 (2009).
Jongsma, M. L. et al. An ER-associated pathway defines endosomal architecture for controlled cargo transport. Cell 166, 152–166 (2016).
Hong, Z. et al. PtdIns3P controls mTORC1 signalling through lysosomal positioning. J. Cell Biol. 216, 4217–4233 (2017).
Chu, B. B. et al. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161, 291–306 (2015).
Starling, G. P. et al. Folliculin directs the formation of a Rab34-RILP complex to control the nutrient-dependent dynamic distribution of lysosomes. EMBO Rep. 17, 823–841 (2016).
Hao, F. et al. Rheb localized on the Golgi membrane activates lysosome-localized mTORC1 at the Golgi-lysosome contact site. J. Cell Sci. 131, jcs208017 (2018).
Wong, Y. C., Ysselstein, D. & Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 (2018).
Henne, W. M. et al. Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. J. Cell Biol. 210, 541–551 (2015).
John Peter, A. T. et al. Vps13-Mcp1 interact at vacuole-mitochondria interfaces and bypass ER-mitochondria contact sites. J. Cell Biol. 216, 3219–3229 (2017).
González Montoro, A. et al. Vps39 interacts with Tom40 to establish one of two functionally distinct vacuole-mitochondria contact sites. Dev. Cell 45, 621–636.e7 (2018).
Cioni, J. M. et al. Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176, 56–72 (2019).
Liao, Y. C. et al. RNA granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164.e20 (2019). Cioni et al. (2019) and Liao et al. (2019) demonstrate that RNA granules ‘hitchhike’ on late endosomes/lysosomes, enabling them to travel along the axon towards sites of local mRNA translation in the proximity of mitochondria, particularly for synthesis of mitochondrial proteins.
Bonifacino, J. S. & Neefjes, J. Moving and positioning the endolysosomal system. Curr. Opin. Cell Biol. 47, 1–8 (2017).
Encarnação, M. et al. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J. Cell Biol. 213, 631–640 (2016).
Nakata, T. & Hirokawa, N. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J. Cell Biol. 131, 1039–1053 (1995).
Harada, A. et al. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 141, 51–59 (1998).
Burkhardt, J. K., Echeverri, C. J., Nilsson, T. & Vallee, R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484 (1997).
Matsushita, M., Tanaka, S., Nakamura, N., Inoue, H. & Kanazawa, H. A novel kinesin-like protein, KIF1Bbeta3 is involved in the movement of lysosomes to the cell periphery in non-neuronal cells. Traffic 5, 140–151 (2004).
Guardia, C. M., Farías, G. G., Jia, R., Pu, J. & Bonifacino, J. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 17, 1950–1961 (2016).
Korolchuk, V. I. et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 13, 453–460 (2011). A landmark article showing that lysosome positioning regulates mTORC1 signalling, autophagosome formation and lysosome–autophagosome fusion.
Li, X. et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 18, 404–417 (2016).
Pu, J. et al. a multiprotein complex that regulates lysosome positioning. Dev. Cell 33, 176–188 (2015).
Filipek, P. A. et al. LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning. J. Cell Biol. 216, 4199–4215 (2017).
Pu, J., Keren-Kaplan, T. & Bonifacino, J. S. A Ragulator-BORC interaction controls lysosome positioning in response to amino acid availability. J. Cell Biol. 216, 4183–4197 (2017). Filipek et al. (2017) and Pu et al. (2017) show that the Ragulator complex engages in a negative regulatory interaction with BORC, mediating perinuclear clustering of lysosomes in response to nutrient deprivation and lysosome dispersal towards the cell periphery in response to epidermal growth factor stimulation.
Raiborg, C. et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 520, 234–238 (2015).
Pu, J., Guardia, C. M., Keren-Kaplan, T. & Bonifacino, J. S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 (2016).
Jia, R. & Bonifacino, J. S. Lysosome positioning influences mTORC2 and AKT signalling. Mol. Cell 75, 26–38.e3 (2019).
Clippinger, A. J. & Alwine, J. C. Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells. Genes. Dev. 26, 2015–2026 (2012).
Walton, Z. E. et al. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell 174, 72–87 (2018).
Clippinger, A. J., Maguire, T. G. & Alwine, J. C. Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. J. Virology 85, 9369–9376 (2011).
Peng, W., Minakaki, G., Nguyen, M. & Krainc, D. Preserving lysosomal function in the ageing brain: insights from neurodegeneration. Neurotherapeutics 16, 611–634 (2019).
Pattison, C. J. & Korolchuk, V. I. Autophagy: ‘self-eating’ your way to longevity. Subcell. Biochem. 90, 25–47 (2018).
Parenti, G., Andria, G. & Ballabio, A. Lysosomal storage diseases: from pathophysiology to therapy. Annu. Rev. Med. 66, 471–486 (2015).
Ballabio, A. & Gieselmann, V. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta 1793, 684–696 (2009).
Marques, A. R. A. & Saftig, P. Lysosomal storage disorders - challenges, concepts and avenues for therapy: beyond rare diseases. J. Cell Sci. 132, jcs221739 (2019).
Platt, F. M. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 17, 133–150 (2018).
Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27 (2018).
Dierks, T. et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C(alpha)-formylglycine generating enzyme. Cell 113, 435–444 (2003).
Cosma, M. P. et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell 113, 445–456 (2003).
di Ronza, A. et al. CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis. Nat. Cell Biol. 20, 1370–1377 (2018).
Kondo, H. et al. Mutation in VPS33A affects metabolism of glycosaminoglycans: a new type of mucopolysaccharidosis with severe systemic symptoms. Hum. Mol. Genet. 26, 173–183 (2017).
Pavlova E. V. et al. The lysosomal disease caused by mutant VPS33A. Hum. Mol. Genet. 28, 2514–2530 (2019).
van der Beek, J., Jonker, C., van der Welle, R. & Liv, N. Klumperman J. CORVET, CHEVI and HOPS - multisubunit tethers of the endo-lysosomal system in health and disease. J. Cell Sci. 132, jcs189134 (2019).
Settembre, C. et al. A block of autophagy in lysosomal storage disorders. Hum. Mol. Genet. 17, 119–129 (2008).
Lieberman, A. P. et al. Autophagy in lysosomal storage disorders. Autophagy 8, 719–730 (2012).
Seranova, E. et al. Dysregulation of autophagy as a common mechanism in lysosomal storage diseases. Essays Biochem. 61, 733–749 (2017).
Fraldi, A. et al. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 29, 3607–3620 (2010).
Bartolomeo, R. et al. mTORC1 hyperactivation arrests bone growth in lysosomal storage disorders by suppressing autophagy. J. Clin. Invest. 127, 3717–3729 (2017). This article demonstrates that bone abnormalities in LSDs are caused by mTORC1 activation and consequent autophagy inhibition that suppresses bone growth.
Lim, J. A. et al. Modulation of mTOR signalling as a strategy for the treatment of Pompe disease. EMBO Mol. Med. 9, 353–370 (2017).
Kinghorn, K. J. et al. A Drosophila model of neuronopathic Gaucher disease demonstrates lysosomal-autophagic defects and altered mTOR signalling and Is functionally rescued by rapamycin. J. Neurosci. 36, 11654–11670 (2016).
Maetzel, D. et al. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick type C patient-specific iPS cells. Stem Cell Rep. 2, 866–880 (2014).
Fraldi, A., Klein, A. D., Medina, D. L. & Settembre, C. Brain disorders due to lysosomal dysfunction. Annu. Rev. Neurosci. 39, 277–295 (2016).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013).
Song, C. Y., Guo, J. F., Liu, Y. & Tang, B. S. Autophagy and its comprehensive impact on ALS. Int. J. Neurosci. 122, 695–703 (2012).
Bras, J. et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum. Mol. Genet. 23, 6139–6146 (2014).
Lee, S. M., Chin, L. S. & Li, L. Charcot-Marie-Tooth disease-linked protein SIMPLE functions with the ESCRT machinery in endosomal trafficking. J. Cell Biol. 199, 799–816 (2012).
BasuRay, S., Mukherjee, S., Romero, E. G., Seaman, M. N. & Wandinger-Ness, A. Rab7 mutants associated with Charcot-Marie-Tooth disease cause delayed growth factor receptor transport and altered endosomal and nuclear signalling. J. Biol. Chem. 288, 1135–1149 (2013).
Lee, J. H. et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158 (2010).
Coen, K. et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 198, 23–35 (2012).
Lee, J. H. et al. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12, 1430–1444 (2015).
Shachar, T. et al. Lysosomal storage disorders and Parkinson disease: Gaucher disease and beyond. Mov. Disord. 26, 1593–1604 (2011).
Robak, L. A. et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson disease. Brain 140, 3191–3203 (2017).
Aflaki, E., Westbroek, W. & Sidransky, E. The complicated relationship between Gaucher disease and parkinsonism: insights from a rare disease. Neuron 93, 737–746 (2017).
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Lee, H. J., Khoshaghideh, F., Patel, S. & Lee, S. J. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 24, 1888–1896 (2004).
Nixon, R. A. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer disease: inseparable partners in a multifactorial disease. FASEB J. 31, 2729–2743 (2017).
Wong, Y. C. & Holzbaur, E. L. The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J. Neurosci. 34, 1293–1305 (2014).
Rui, Y. N. et al. Huntingtin functions as a scaffold for selective macroautophagy. Nat. Cell Biol. 17, 262–275 (2015).
Martinez-Vicente, M. et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington disease. Nat. Neurosci. 13, 567–576 (2010).
Freeman, D. et al. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLOS ONE 8, e62143 (2013).
Ying, J. et al. Lysosomal dysfunction in Down syndrome is APP-dependent and mediated by APP-βCTF (C99). J. Neurosci. 39, 5255–5268 (2019).
Gowrishankar, S. et al. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer disease amyloid plaques. Proc. Natl Acad. Sci. USA 112, E3699–E3708 (2015).
Martini-Stoica, H., Xu, Y., Ballabio, A. & Zheng, H. The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci. 39, 221–234 (2016).
Boya, P. & Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 27, 6434–6451 (2008).
Appelqvist, H., Wäster, P., Kågedal, K. & Öllinger, K. The lysosome: from waste bag to potential therapeutic target. J. Mol. Cell Biol. 5, 214–226 (2013).
Kimmelman, A. C. & White, E. Autophagy and tumour metabolism. Cell Metab. 25, 1037–1043 (2017).
Perera, R. M. et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 524, 361–365 (2015).
Perera, R. M., Di Malta, C. & Ballabio, A. MiT/TFE family of transcription factors, lysosomes, and cancer. Annu. Rev. Cancer Biol. 3, (203–222 (2019).
Glunde, K. et al. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 5, 533–545 (2003).
Bian, B. et al. Cathepsin B promotes colorectal tumorigenesis, cell invasion, and metastasis. Mol. Carcinog. 55, 671–687 (2016).
Monteiro, P. et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J. Cell Biol. 203, 1063–1079 (2013).
Dozynkiewicz, M. A. et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 22, 131–145 (2012).
Ylivinkka, I. et al. Motility of glioblastoma cells is driven by netrin-1 induced gain of stemness. J. Exp. Clin. Cancer Res. 36, 9 (2017).
Circu, M. L. et al. A novel high content imaging-based screen identifies the anti-helminthic niclosamide as an inhibitor of lysosome anterograde trafficking and prostate cancer cell invasion. PLOS ONE 11, e0146931 (2016).
Jaishy, B. & Abel, E. D. Lipids, lysosomes, and autophagy. J. Lipid. Res. 57, 1619–1635 (2016).
Mészáros, G., Pasquier, A., Vivot, K., Goginashvili, A. & Ricci, R. Lysosomes in nutrient signalling: a focus on pancreatic β-cells. Diabetes Obes. Metab. 20, 104–115 (2018).
Gilleron, J., Gerdes, J. M. & Zeigerer, A. Metabolic regulation through the endosomal system. Traffic 20, 552–570 (2019).
Gornicka, A. et al. Adipocyte hypertrophy is associated with lysosomal permeability both in vivo and in vitro: role in adipose tissue inflammation. Am. J. Physiol. Endocrinol. Metab. 303, E597–E606 (2012).
Goginashvili, A. et al. Insulin secretory granules control autophagy in pancreatic β cells. Science 347, 878–882 (2015). Starvation of pancreatic β-cells is shown to induce direct fusion of lysosomes with nascent secretory insulin granules by a process that is distinct from conventional autophagy and is controlled by protein kinase D.
Chu, K. Y., O’Reilly, L., Ramm, G. & Biden, T. J. High-fat diet increases autophagic flux in pancreatic beta cells in vivo and ex vivo in mice. Diabetologia 58, 2074–2078 (2015).
Knapp, P. E. & Swanson, J. A. Plasticity of the tubular lysosomal compartment in macrophages. J. Cell Sci. 95, 433–439 (1990).
Lee, S., Sato, Y. & Nixon, R. A. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J. Neurosci. 31, 7817–7830 (2011).
Johnson, D. E., Ostrowski, P., Jaumouille, V. & Grinstein, S. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 212, 677–692 (2016). This study highlights the heterogeneity of lysosomes by demonstrating that peripherally localized lysosomes have higher pH and lower proteolytic activity than their perinuclearly localized counterparts.
Johnson A. E., Shu H., Hauswirth A. G., Tong A., Davis G. W. VCP-dependent muscle degeneration is linked to defects in a dynamic tubular lysosomal network in vivo. eLife 4, e07366 (2015).
Mrakovic, A., Kay, J. G., Furuya, W., Brumell, J. H. & Botelho, R. J. Rab7 and Arl8 GTPases are necessary for lysosome tubulation in macrophages. Traffic 13, 1667–1679 (2012).
Rubinsztein, D. C., Codogno, P. & Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 11, 709–730 (2012).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Yamamoto, S. et al. Autophagy differentially regulates insulin production and insulin sensitivity. Cell Rep. 23, 3286–3299 (2018).
Raiborg C. How nutrients orchestrate lysosome positioning. Contact https://doi.org/10.1177/2515256418756111 (2018).
Ferron, M. et al. A RANKL-PKCβ-TFEB signalling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev. 27, 955–969 (2013).
El-Houjeiri, L. et al. The transcription factors TFEB and TFE3 link the FLCN-AMPK signalling axis to innate immune response and pathogen resistance. Cell Rep. 26, 3613–3628.e6 (2019).
Doronzo, G. et al. TFEB controls vascular development by regulating the proliferation of endothelial cells. EMBO J. 38, e98250 (2019).
Fan, Y. et al. Endothelial TFEB (transcription factor EB) positively regulates postischemic angiogenesis. Circ. Res. 122, 945–957 (2018).
Wada, S. et al. The tumour suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 30, 2551–2564 (2016).
Murano, T. et al. Transcription factor TFEB cell-autonomously modulates susceptibility to intestinal epithelial cell injury in vivo. Sci. Rep. 7, 13938 (2017).
Meireles, A. M. et al. The lysosomal transcription factor TFEB represses myelination downstream of the Rag-Ragulator complex. Dev. Cell 47, 319–330.e5 (2018).
Acknowledgements
The authors thank A. De Matteis, G. Diez-Roux, P. Luzio, R. Mattera, G. Napolitano and C. Settembre for comments on the manuscript and J. Goodwin for the original draft of Fig. 3a. Work in A.B.’s laboratory is supported by the US National Institutes of Health (R01-NS078072), the Italian Association for Cancer Research (AIRC) (IG 2018 22103), Fondation Louis-Jeantet and the Telethon Foundation. Work in J.S.B.’s laboratory is supported by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (ZIA HD001607).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
A.B. is a co-founder of CASMA Therapeutics (Boston, MA, USA). J.S.B. declares no competing interest.
Additional information
Peer review informationNature Reviews Molecular Cell Biology thanks R. Puertollano, S. Ferguson, J. Klumperman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Membrane contact sites
-
Membrane domains where organelles are closely (10–30 nm) held together by tethering proteins.
- RAG GTPases
-
RAGA, RAGB, RAGC and RAGD are small GTPases that belong to a subfamily of the RAS-related GTPases. They act as heterodimers in which RAGA or RAGB interacts with RAGC or RAGD. Their activation by amino acids mediates the recruitment of mechanistic target of rapamycin complex 1 to the lysosomal surface.
- Ragulator
-
Protein complex composed of five subunits (LAMTOR1–LAMTOR5). It is located on the lysosomal surface, where it interacts with RAG GTPases for the recruitment of mechanistic target of rapamycin complex 1 to the lysosome. It also interacts with BORC for the regulation of lysosome positioning.
- Tuberous sclerosis complex
-
(TSC). Protein complex, composed of TSC1 (hamartin), TSC2 (tuberin) and TBC1D7, which functions as a GTPase-activating protein for the small GTPase RHEB.
- Folliculin
-
(FLCN). Tumour-suppressor protein that exists as part of a complex with the folliculin-interacting protein and functions as a GTPase-activating protein for the RAGC and RAGD GTPases.
- Endolysosomes
-
Generic term for various types of endosomes and lysosomes.
- Sphingosine
-
Long-chain unsaturated amino alcohol that forms the backbone of a class of membrane lipids known as sphingolipids.
- Phosphoinositide
-
A class of phospholipids comprising a myo-inositol head group linked by a glycerol moiety to two fatty acyl chains. Phosphoinositides are minor components of cellular membranes involved in signalling and regulation of membrane dynamics.
- 14-3-3 proteins
-
Family of proteins that bind phosphorylated serine or threonine residues on various regulatory proteins such as kinases, phosphatases, transcription factors and signal-transduction proteins.
- Hyperuricaemia
-
Elevated levels of uric acid in blood.
- Lysosomotropic drugs
-
Drugs that concentrate within the acidic lumen of lysosomes and modify lysosomal function.
- Pyroptosis
-
A type of programmed cell death most often triggered by infection with intracellular pathogens and mediated by pore-forming gasdermin D, which permeabilizes the plasma membrane.
- Ferroptosis
-
Iron-dependent form of cell death triggered by inactivation of cellular glutathione-dependent antioxidant defences with consequent accumulation of lipid reactive oxygen species.
- Necroptosis
-
A programmed form of necrosis that is downstream of cell-death receptor signalling, which is generally induced by cell damage but can also be promoted by lysosomal membrane permeabilization.
- Galectin protein family
-
Endogenous carbohydrate-binding proteins (lectins) with specificity for β-galactoside sugars.
- Tripartite motif (TRIM) protein family
-
Family of E3 ubiquitin ligases having RING, B-box and coiled-coil domains.
- AMPK
-
AMP-activated protein kinase that functions as a master regulator of cellular energy metabolism.
- ESCRT machinery
-
Ensemble of complexes known as endosomal sorting complexes required for transport that perform various membrane bending and scission reactions away from the cytoplasm.
- Toll-like receptor (TLR) family
-
Family of transmembrane receptors that recognize pathogen-associated molecular patterns as part of the innate immune response.
- Pathogen-associated molecular patterns
-
Microbial molecules that trigger innate immune responses.
- Nuclear factor-κB
-
Family of transcription factors that regulate the expression of genes involved in both innate and adaptive immunity.
- Interferon regulatory factors
-
Transcription factors that control both innate and adaptive immunity against invading pathogens.
- SNAREs
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors, which orchestrate membrane fusion events in the cytoplasm.
- Oleoylethanolamide
-
(OEA). Monounsaturated fatty acid ethanolamide that functions as a bioactive lipid in many physiological processes.
- Nuclear hormone receptors
-
Ligand-activated receptors that bind to specific DNA sequences and regulate gene transcription.
- β-oxidation
-
Catabolic process by which fatty acids are broken down to generate acetyl-CoA in mitochondria.
- MiT-TFE family
-
Family of helix–loop–helix leucine zipper transcription factors that regulate expression of genes involved in the biogenesis and function of lysosomes and autophagosomes.
- Unfolded protein response
-
Cellular stress response triggered by accumulation of unfolded proteins in the endoplasmic reticulum.
- Spermidine
-
Organic polycation that regulates various cellular processes, including translation.
- Vacuolar ATPase
-
(v-ATPase). Multisubunit, ATP-driven proton pump responsible for the acidification of intracellular compartments.
- Tethering complex
-
Protein complex that promotes SNARE-dependent fusion of membrane-bound organelles.
- Atg8/LC3/GABARAP family
-
Homologues of yeast autophagy-related protein 8 (Atg8); ubiquitin-like proteins that function in cargo recruitment to autophagosomes and autophagosome–lysosome fusion.
- RHEB
-
Member of the RAS family of small GTPases that is mainly involved in activation of mechanistic target of rapamycin complex 1.
- Retrograde movement
-
In general, movement from the plus end to the minus end of microtubules; in the axon, movement from the axon terminal towards the cell body.
- Anterograde movement
-
In general, movement from the minus end to the plus end of microtubules; in the axon, movement from the cell body towards the axon terminal.
- CORVET
-
Tethering complex composed of six subunits (VPS3, VPS8, VPS11, VPS16, VPS18 and VPS33) that functions in endosomal fusion events.
- Neurofibrillary tangles
-
Intraneuronal aggregates of hyperphosphorylated tau protein that are most commonly associated with Alzheimer disease.
- α-Synuclein
-
Presynaptic protein pathogenetically linked to Parkinson disease.
- Pompe disease
-
Lysosomal storage disorder caused by acid α-glucosidase deficiency, which results in accumulation of glycogen in the cells.
- Gaucher disease
-
Lysosomal storage disorder caused by deficiency of glucocerebrosidase, which results in the build-up of glucocerebroside in the cells.
- Lewy bodies
-
Abnormal deposits of the protein α-synuclein in the brain of patients with various neurodegenerative disorders, including Lewy body dementia, Parkinson disease and Alzheimer disease.
- Presenilin 1
-
One of the four subunits of the γ-secretase complex. The encoding gene is mutated in an inherited form of Alzheimer disease.
- Amyloid precursor protein
-
(APP). Transmembrane protein that is proteolytically processed to various products, including amyloid-β peptide involved in Alzheimer disease.
- mTORC2
-
Protein complex comprising the mechanistic target of rapamycin kinase and five additional subunits (MLST8, DEPTOR, MSIN1, PROTOR and RICTOR) that functions in the regulation of cellular metabolism and growth.
- Netrin
-
Secreted protein that functions in cell migration and cell–cell and cell–extracellular matrix interactions.
- Anchor cells
-
Cells that participate in the development of the reproductive system in Caenorhabditis elegans.
Rights and permissions
About this article
Cite this article
Ballabio, A., Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol 21, 101–118 (2020). https://doi.org/10.1038/s41580-019-0185-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-019-0185-4
This article is cited by
-
Low-intensity pulsed ultrasound delays the progression of osteoarthritis by regulating the YAP–RIPK1–NF-κB axis and influencing autophagy
Journal of Translational Medicine (2024)
-
A SPLICS reporter reveals \({{{{{\boldsymbol{\alpha }}}}}}\)-synuclein regulation of lysosome-mitochondria contacts which affects TFEB nuclear translocation
Nature Communications (2024)
-
Content-aware frame interpolation (CAFI): deep learning-based temporal super-resolution for fast bioimaging
Nature Methods (2024)
-
Regulation of autophagy by perilysosomal calcium: a new player in β-cell lipotoxicity
Experimental & Molecular Medicine (2024)
-
Advanced oxidation protein products attenuate the autophagy-lysosome pathway in ovarian granulosa cells by modulating the ROS-dependent mTOR-TFEB pathway
Cell Death & Disease (2024)