Abstract
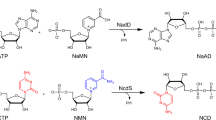
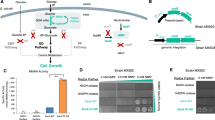
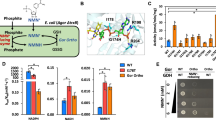
Biological production of chemicals often requires the use of cellular cofactors, such as nicotinamide adenine dinucleotide phosphate (NADP+). These cofactors are expensive to use in vitro and difficult to control in vivo. We demonstrate the development of a noncanonical redox cofactor system based on nicotinamide mononucleotide (NMN+). The key enzyme in the system is a computationally designed glucose dehydrogenase with a 107-fold cofactor specificity switch toward NMN+ over NADP+ based on apparent enzymatic activity. We demonstrate that this system can be used to support diverse redox chemistries in vitro with high total turnover number (~39,000), to channel reducing power in Escherichia coli whole cells specifically from glucose to a pharmaceutical intermediate, levodione, and to sustain the high metabolic flux required for the central carbon metabolism to support growth. Overall, this work demonstrates efficient use of a noncanonical cofactor in biocatalysis and metabolic pathway design.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all relevant data supporting the findings of this study are available within the manuscript and its Supplementary Information.
References
Lee, S. Y. & Kim, H. U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 33, 1061–1072 (2015).
Mampel, J., Buescher, J. M., Meurer, G. & Eck, J. Coping with complexity in metabolic engineering. Trends Biotechnol. 31, 52–60 (2013).
Paddon, C. J. & Keasling, J. D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 12, 355 (2014).
Pandit, A. V., Srinivasan, S. & Mahadevan, R. Redesigning metabolism based on orthogonality principles. Nat. Commun. 8, 15188 (2017).
Wang, L. et al. Synthetic cofactor-linked metabolic circuits for selective energy transfer. ACS Catal. 7, 1977–1983 (2017).
Martínez, A. T. et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 35, 815–831 (2017).
Wildeman, S. M. A. D., Sonke, T., Schoemaker, H. E. & May, O. Biocatalytic reductions: from lab curiosity to ‘first choice’. Acc. Chem. Res. 40, 1260–1266 (2007).
Campbell, E., Meredith, M., Minteer, S. D. & Banta, S. Enzymatic biofuel cells utilizing a biomimetic cofactor. Chem. Commun. 48, 1898–1900 (2012).
Knaus, T. et al. Better than Nature: nicotinamide biomimetics that outperform natural coenzymes. J. Am. Chem. Soc. 138, 1033–1039 (2016).
Nowak, C., Pick, A., Lommes, P. & Sieber, V. Enzymatic reduction of nicotinamide biomimetic cofactors using an engineered glucose dehydrogenase: providing a regeneration system for artificial cofactors. ACS Catal. 7, 5202–5208 (2017).
Flores, H. & Ellington, A. D. A modified consensus approach to mutagenesis inverts the cofactor specificity of Bacillus stearothermophilus lactate dehydrogenase. Protein Eng. Des. Sel. 18, 369–377 (2005).
Lo, H. C. & Fish, R. H. Biomimetic NAD(+) models for tandem cofactor regeneration, horse liver alcohol dehydrogenase recognition of 1,4-NADH derivatives, and chiral synthesis. Angew. Chem. 41, 478–481 (2002).
Hagen, J. Industrial Catalysis: A Practical Approach 2nd edn (Wiley-VCH, 2006).
Everse, J., Anderson, B. & You, K.-S. The Pyridine Nucleotide Coenzymes (Academic Press, 1982).
Paul, C. E., Arends, I. W. C. E. & Hollmann, F. Is simpler better? Synthetic nicotinamide cofactor analogues for redox chemistry. ACS Catal. 4, 788–797 (2014).
Paul, C. E. et al. Mimicking nature: synthetic nicotinamide cofactors for C=C bioreduction using enoate reductases. Org. Lett. 15, 180–183 (2013).
Kataoka, M. et al. Old Yellow Enzyme from Candida macedoniensis catalyzes the stereospecific reduction of the C=C bond of ketoisophorone. Biosci. Biotechnol. Biochem. 66, 2651–2657 (2002).
Okamoto, Y., Köhler, V., Paul, C. E., Hollmann, F. & Ward, T. R. Efficient in situ regeneration of NADH mimics by an artificial metalloenzyme. ACS Catal. 6, 3553–3557 (2016).
Chaparro-Riggers, J. F., Rogers, T. A., Vazquez-Figueroa, E., Polizzi, K. M. & Bommarius, A. S. Comparison of three enoate reductases and their potential use for biotransformations. Adv. Synth. Catal. 349, 1521–1531 (2007).
Paul, C. E. et al. Nonenzymatic regeneration of styrene monooxygenase for catalysis. ACS Catal. 5, 2961–2965 (2015).
Knox, R. J. et al. Virtual cofactors for an Escherichia coli nitroreductase enzyme: relevance to reductively activated prodrugs in antibody directed enzyme prodrug therapy (ADEPT). Biochem. Pharmacol. 49, 1641–1647 (1995).
Ryan, J. D., Fish, R. H. & Clark, D. S. Engineering cytochrome P450 enzymes for improved activity towards biomimetic 1,4-NADH cofactors. Chem. Bio. Chem. 9, 2579–2582 (2008).
Müller, A., Stürmer, R., Hauer, B. & Rosche, B. Stereospecific alkynereduction: novel activity of old yellow enzymes. Angew. Chem. Int. Ed. 46, 3316–3318 (2007).
Race, P. R. et al. Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone. Reversed binding orientations in different redox states of the enzyme. J. Biol. Chem. 280, 13256–13264 (2005).
Hilt, W., Pfleiderer, G. & Fortnagel, P. Glucose dehydrogenase from Bacillus subtilis expressed in Escherichia coli. I: Purification, characterization and comparison with glucose dehydrogenase from Bacillus megaterium. Biochim. Biophys. Acta 1076, 298–304 (1991).
Solanki, K., Abdallah, W. & Banta, S. Engineering the cofactor specificity of an alcohol dehydrogenase via single mutations or insertions distal to the 2′-phosphate group of NADP(H). Protein Eng. Des. Sel. 30, 373–380 (2017).
Schewe, H., Kaup, B. A. & Schrader, J. Improvement of P450(BM-3) whole-cell biocatalysis by integrating heterologous cofactor regeneration combining glucose facilitator and dehydrogenase in E. coli. Appl. Microbiol. Biotechnol. 78, 55–65 (2008).
Pohlmann, A. et al. Genome sequence of the bioplastic-producing ‘Knallgas’ bacterium Ralstonia eutropha H16. Nat. Biotechnol. 24, 1257–1262 (2006).
Gazzaniga, F., Stebbins, R., Chang, S. Z., McPeek, M. A. & Brenner, C. Microbial NAD metabolism: lessons from comparative genomics. Microbiol. Mol. Biol. Rev. 73, 529–541 (2009).
Sorci, L. et al. Nicotinamide mononucleotide synthetase is the key enzyme for an alternative route of NAD biosynthesis in Francisella tularensis. Proc. Natl Acad. Sci. USA 106, 3083–3088 (2009).
Stott, K., Saito, K., Thiele, D. J. & Massey, V. Old Yellow Enzyme. The discovery of multiple isozymes and a family of related proteins. J. Biol. Chem. 268, 6097–6106 (1993).
Wang, X. et al. Engineering Escherichia coli nicotinic acid mononucleotide adenylyltransferase for fully active amidated NAD biosynthesis. Appli. Environ. Microbiol. 83, e00692-17 (2017).
Witholt, B. Method for isolating mutants overproducing nicotinamide adenine dinucleotide and its precursors. J. Bacteriol. 109, 350–364 (1972).
Grozio, A. et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 1, 47–57 (2019).
Grose, J. H. et al. Assimilation of nicotinamide mononucleotide requires periplasmic AphA phosphatase in Salmonella enterica. J. Bacteriol. 187, 4521–4530 (2005).
Kunjapur, A. M. & Prather, K. L. Microbial engineering for aldehyde synthesis. Appl. Environ. Microbiol. 81, 1892–1901 (2015).
Rodriguez, G. M. & Atsumi, S. Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab. Eng. 25, 227–237 (2014).
Hall, M., Hauer, B., Stuermer, R., Kroutil, W. & Faber, K. Asymmetric whole-cell bioreduction of an α,β-unsaturated aldehyde (citral): competing prim-alcohol dehydrogenase and C–C lyase activities. Tetrahedron Asymmetry 17, 3058–3062 (2006).
Yoshisumi, A., Wada, M., Takagi, H., Shimizu, S. & Nakamori, S. Cloning, sequence analysis, and expression in Escherichia coli of the gene encoding monovalent cation-activated levodione reductase from Corynebacterium aquaticum M-13. Biosci. Biotechnol. Biochem. 65, 830–836 (2001).
Kulig, J., Frese, A., Kroutil, W., Pohl, M. & Rother, D. Biochemical characterization of an alcohol dehydrogenase from Ralstonia sp. Biotechnol. Bioeng. 110, 1838–1848 (2013).
Oberleitner, N., Peters, C., Rudroff, F., Bornscheuer, U. T. & Mihovilovic, M. D. In vitro characterization of an enzymatic redox cascade composed of an alcohol dehydrogenase, an enoate reductases and a Baeyer–Villiger monooxygenase. J. Biotechnol. 192 Pt B, 393–399 (2014).
Mak, W. S. et al. Integrative genomic mining for enzyme function to enable engineering of a non-natural biosynthetic pathway. Nat. Commun. 6, 10005 (2015).
Stewart, J. D. in Future Directions in Biocatalysis (ed. T. Matsuda) Ch. 12 (Elsevier Science, 2007).
Liang, K. & Shen, C. R. Selection of an endogenous 2,3-butanediol pathway in Escherichia coli by fermentative redox balance. Metab. Eng. 39, 181–191 (2017).
Machado, H. B., Dekishima, Y., Luo, H., Lan, E. I. & Liao, J. C. A selection platform for carbon chain elongation using the CoA-dependent pathway to produce linear higher alcohols. Metab. Eng. 14, 504–511 (2012).
Zhang, L., King, E., Luo, R. & Li, H. Development of a high-throughput, in vivo selection platform for NADPH-dependent reactions based on redox balance principles. ACS Synth. Biol. 7, 1715–1721 (2018).
Marinescu, G. C., Popescu, R. G., Stoian, G. & Dinischiotu, A. Beta-nicotinamide mononucleotide (NMN) production in Escherichia coli. Sci. Rep. 8, 12278 (2018).
Finn, R. D. et al. HMMER web server: 2015 update. Nucleic Acids Res. 43, W30–W38 (2015).
Yamamoto, K. et al. Crystal structure of glucose dehydrogenase from Bacillus megaterium IWG3 at 1.7 A resolution. J. Biochem. 129, 303–312 (2001).
Song, Y. et al. High-resolution comparative modeling with RosettaCM. Structure 21, 1735–1742 (2013).
Richter, F., Leaver-Fay, A., Khare, S. D., Bjelic, S. & Baker, D. De novo enzyme design using Rosetta3. PloS One 6, e19230 (2011).
Kleffner, R. et al. Foldit Standalone: a video game-derived protein structure manipulation interface using Rosetta. Bioinformatics 33, 2765–2767 (2017).
Shao, Y. et al. Spartan’08, Wavefunction, Inc. Irvine, CA. Phys. Chem. Chem. Phys. 8, 3172–3191 (2006).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343 (2009).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Findik, B. T. & Randall, L. L. Determination of the intracellular concentration of the export chaperone SecB in Escherichia coli. PLoS One 12, e0183231 (2017).
Fischer, J., Holliday, G. & Thornton, J. The CoFactor database: organic cofactors in enzyme catalysis. Bioinformatics 26, 2496–2497 (2010).
Acknowledgements
H.L. acknowledges support from University of California, Irvine, the National Science Foundation (NSF) (award no. 1847705), and the National Institutes of Health (NIH) (award no. DP2 GM137427). S.M. acknowledges support from the NSF Graduate Research Fellowship Program (grant no. DGE-1839285). W.B.B. acknowledges support from Graduate Assistance in Areas of National Need fellowship funded by the U.S. Department of Education. J.B.S., W.S.M. and Y.C. acknowledge support from the University of California, Davis, by the NSF (award nos. 1827246, 1805510, 1627539), the National Institute of Environmental Health Sciences of the NIH (award no. P42ES004699) and the NIH (award no. R01 GM 076324-11). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the NSF. We thank the University of California, Irvine Mass Spectrometry Facility and F. Grun for help with LC–MS analysis.
Author information
Authors and Affiliations
Contributions
H.L. and J.B.S. conceived the research. L.Z., W.B.B., and E.K. performed mutant enzyme kinetics characterization. W.B.B., L.Z., S.M., E.K., and B.F. performed the in vitro biotransformation. W.B.B., S.M., B.F., and A.S.M. performed the whole-cell biotransformation. W.B.B. performed the intracelluar NMN+ and NAD+ level analysis. S.M. performed the NMN+-dependent growth experiments. W.S.M. and Y.C. performed the computational modeling. All authors analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and Figs. 1–8.
Rights and permissions
About this article
Cite this article
Black, W.B., Zhang, L., Mak, W.S. et al. Engineering a nicotinamide mononucleotide redox cofactor system for biocatalysis. Nat Chem Biol 16, 87–94 (2020). https://doi.org/10.1038/s41589-019-0402-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0402-7
This article is cited by
-
How synthetic biologists are building better biofactories
Nature (2024)
-
Enhancing nicotinamide mononucleotide production from glucose in Escherichia coli by genetic engineering
Systems Microbiology and Biomanufacturing (2024)
-
Redox signaling-driven modulation of microbial biosynthesis and biocatalysis
Nature Communications (2023)
-
Enzymatic synthesis of high-titer nicotinamide mononucleotide with a new nicotinamide riboside kinase and an efficient ATP regeneration system
Bioresources and Bioprocessing (2022)
-
A phosphite-based screening platform for identification of enzymes favoring nonnatural cofactors
Scientific Reports (2022)