Abstract
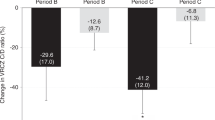
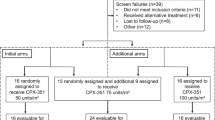
To optimize voriconazole dosing in pediatric hematopoietic cell transplantation (HCT), we conducted a phase I study with a modified 3 + 3 dose-escalation followed by an expansion cohort at the maximum tolerated, minimum efficacious dose (MTD/MED). Patients ≤21 years who required voriconazole for prevention or treatment of an invasive fungal infection were assigned to three age groups. Of the 59 evaluable patients, 13 were <2 years, 23 were 2–11, and 23 were 12–21. Therapeutic serum voriconazole troughs (1.5–5 µg/mL) drawn at 7 days after initiation determined efficacy. The MTD/MED was 12 mg/kg/dose q12 h × 2 loading doses, then 10 mg/kg/dose q12 h in patients <2, and was 10 mg/kg/dose q12 h in patients 2–11. The 12–21 age group had no dose-limiting toxicity at 8 mg/kg/dose q12 h; however, the MED was not reached. Drug-related AEs ≥grade 3 included increased bilirubin, transaminases, and creatinine, all occurring in <10%. There was no significant association between supra-therapeutic troughs and AEs. Five of 17 patients who had supra-therapeutic troughs (29%) had an AE, compared to 8 of 42 who did not (19%, p = 0.38). Observational population pharmacokinetic analysis demonstrated that inter-individual variability on voriconazole clearance was >100% CV, and clearance increased with age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, Nania JJ, et al. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics. 2008;121:e1286–94.
Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–60.
Antachopoulos C, Walsh TJ, Roilides E. Fungal infections in primary immunodeficencies. Eur J Pediatr. 2007;166:1099–117.
Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transpl. 2009;44:361–70.
Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:2055–61.
van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transpl. 2007;13:1487–98.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transpl. 2009;15:1143–238.
Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2005;36:621–9.
Hovi L, Saarinen-Pihkala UM, Vettenranta K, Saxen H. Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transpl. 2000;26:999–1004.
Trifilio S, Singhal S, Williams S, Frankfurt O, Gordon L, Evens A, et al. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transpl. 2007;40:451–6.
Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010;16:927–33.
Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55:1080–7.
Chu HY, Jain R, Xie H, Pottinger P, Fredricks DN. Voriconazole therapeutic drug monitoring: retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect Dis. 2013;13:105.
Cronin S, Chandrasekar P. Safety of triazole antifungal drugs in patients with cancer. Antimicrob Chemother. 2010;65:410–6.
Trifilio S, Scheetz M, Pi J, Mehta J. Tacrolimus use in adult allogeneic stem cell transplant recipients receiving voriconazole: preemptive dose modification and therapeutic drug monitoring. Bone Marrow Transpl. 2010;45:1352–6.
Marty F, Lowry C, Cutler CS, Campbell BJ, Fiumara K, Baden LR, et al. Voriconazole and sirolimus coadministration after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2006;12:552–9.
Hicks JK, Crews KR, Flynn P, et al. Voriconazole plasma concentrations in immunocompromised pediatric patients vary by CYP2C19 diplotypes. Pharmacogenomics. 2014;15:1065–78.
Kim SH, Lee DG, Kwon JC, Lee HJ, Cho SY, Park C, et al. Clinical impact of cytochrome P450 2C19 genotype on the treatment of invasive aspergillosis under routine therapeutic drug monitoring of voriconazole in a Korean population. Infect Chemother. 2013;45:406–14.
Teusink A, Vinks A, Zhang K, Davies S, Fukuda T, Lane A, et al. Genotype-directed dosing leads to optimized voriconazole levels in pediatric patients receiving hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2016;22:482–6.
Soler-Palacin P, Frick MA, Martin-Nalda A, Lanaspa M, Pou L, Roesllo E, et al. Voriconazole drug monitoring in the management of invasive fungal infection in immunocompromised children: a prospective study. J Antimicrob Chemother. 2012;67:700–6.
Karlsson M, Lutsar I, Milligan P. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob Agents Chemother. 2009;53:935–44.
Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin Infect Dis. 2010;50:27–36.
Shima H, Miharu M, Osumi T, Takahashi T, Shimada H. Differences in voriconazole trough plasma concentrations per oral dosages between children younger and older than 3 years of age. Pediatr Blood Cancer. 2010;54:1050–2.
Michael C, Bierbach U, Frenzel K, Lange T, Basara N, Niederwieser D, et al. Voriconazole pharmacokinetics and safety in immunocompromised children compared to adult patients. Antimicrob Agents Chemother. 2010;54:3225–32.
Friberg LE, Ravva P, Karlsson MO, Liu P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob Agents Chemother. 2012;56:3032–42.
Decosterd LA, Rochat B, Pesse B, Mercier T, Tissot F, Widmer N, et al. Multiplex ultra-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification in human plasma of fluconazole, itraconazole, hydroxyitraconazole, posaconazole, voriconazole, voriconazole-N-oxide, anidulafungin, and caspofungin. Antimicrob Agents Chemother. 2010;54:5303–15.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacomet Syst Pharmacol. 2014;3:e150.
Johnson T, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants, and children. Clin Pharmacokinet. 2006;45:931–56.
Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7.
Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK Jr, Thakker DR. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab Dispos. 2010;38:25–31.
Lee S, Kim BH, Nam WS, Yoon SH, Cho JY, Shin SG, et al. Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2012;52:195–203.
Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49:196–204.
Scholz I, Oberwittler H, Riedel KD, Burhenne J, Weiss J, Haefeli WE, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol. 2009;68:906–15.
Wang G, Lei HP, Li Z, Tan ZR, Guo D, Fan L, et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol. 2009;65:281–5.
Acknowledgements
We would like to acknowledge the assistance of the Clinical Pharmacology Shared Resource of the Masonic Cancer Center, designated by the National Cancer Institute, supported in part by P30 CA77598.
Funding
This work was supported by the Hematology Oncology Pharmacist Association (HOPA) Foundation (MNK), and the Department of Pediatrics, Division of Blood and Marrow Transplant, University of Minnesota (ARS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Knight-Perry, J., Jennissen, C., Long, S.E. et al. A phase I dose finding study of intravenous voriconazole in pediatric patients undergoing hematopoietic cell transplantation. Bone Marrow Transplant 55, 955–964 (2020). https://doi.org/10.1038/s41409-019-0757-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0757-0
This article is cited by
-
Clinical Pharmacokinetics of Triazoles in Pediatric Patients
Clinical Pharmacokinetics (2021)