Abstract
In this work, the Homotopy Perturbation method is used for the first time to solve an irreversible linear pathway with enzyme kinetics. The enzymatic system has Michaelis–Menten kinetics and is modeled by a system of nonlinear ordinary differential equations. The analytical solution obtained with the method allow us to optimize several objectives: minimal time to reach a certain percent of final product, minimal amount of enzymes employed in the process, or even multiple objective optimization via Pareto front. We present an example to demonstrate the results.





Similar content being viewed by others
References
L. Rajendran, M.C. Devi, C. Fernandez, Q. Peng, in Mathematical Modeling and Simulation of Nonlinear Process in Enzyme Kinetics in Advanced Chemical Kinetics, ed. by M. Akhyar Farrukh (InTech, Rijeka, 2018), p. 21
A. Meena, A. Eswari, L. Rajendran, Mathematical modelling of enzyme kinetics reaction mechanisms and analytical solutions of non-linear reaction equations. J. Math. Chem. 48(2), 179–186 (2010)
R. Heinrich, S.M. Rapoport, T.A. Rapoport, Metabolic regulation and mathematical models. Progr. Biophys. Mol. Biol. 32, 1–82 (1977)
K. Bhattacharyya, S. Dhatt, Enzyme kinetics: a critique of the quasi-steady-state approximation (2013). arXiv preprint arXiv:1305.0929
J.A.M. Borghans, R.J. De Boer, L.A. Segel, Extending the quasi-steady state approximation by changing variables. Bull. Math. Biol. 58, 43–63 (1996)
H.P. Kasserra, K.J. Laidler, Transient-phase studies of a trypsin-catalyzed reaction. Can. J. Chem. 48, 1793–1802 (1970)
S. Schnell, Validity of the Michaelis–Menten equation—steady-state or reactant stationary assumption: that is the question. FEBS J. 281, 464–472 (2014)
M.U. Maheswari, L. Rajendran, Analytical solution of non-linear enzyme reaction equations arising in mathematical chemistry. J. Math. Chem. 49(8), 1713 (2011)
G. Varadharajan, L. Rajendran, Analytical solution of coupled non-linear second order reaction differential equations in enzyme kinetics. Nat. Sci. 3(6), 459–465 (2011)
S. Khoshnaw, Iterative approximate solutions of kinetic equations for reversible enzyme reactions (2012). arXiv preprint arXiv:1208.0747
A. Alawneh, Application of the multistep generalized differential transform method to solve a time-fractional enzyme kinetics. Discrete Dyn. Nat. Soc. (2013). https://doi.org/10.1155/2013/592938
G. de Hijas-Liste, E. Klipp, E. Balsa-Canto, J. Banga, Global dynamic optimization approach to predict activation in metabolic pathways. BMC Syst. Biol. 8(1), 1–15 (2014)
J.H. He, Homotopy perturbation technique. Comput. Math. Appl. Mech. Eng. 178, 257–262 (1999)
J.H. He, Homotopy perturbation method: a new nonlinear analytical Technique. Appl. Math. Comput. 135, 73–79 (2003)
M.A. Savageau, Michaelis–Menten mechanism reconsidered: implications of fractal kinetics. J. Theor. Biol. 176(1), 115–124 (1995)
L. Bayón, J.A. Otero, P.M. Suárez, C. Tasis, Solving linear unbranched pathways with Michaelis–Menten kinetics using the Lambert W-function. J. Math. Chem. 54(7), 1351–1369 (2016)
L. Menten, M.I. Michaelis, Die kinetik der invertinwirkung. Biochem Z. 49, 333–369 (1913)
S. Schnell, P.K. Maini, Enzyme kinetics at high enzyme concentration. Bull. Math. Biol. 62, 483–499 (2000)
A. Sillero, V. García-Herrero, Theoretical evaluation of both unknown substrate concentrations and enzyme kinetic constants of metabolic cycles. J. Biomed. Sci. Eng. 8(08), 479 (2015)
R.T. Marler, J.S. Arora, The weighted sum method for multi-objective optimization: new insights. Struct. Multidisc. Optim. 41, 853–862 (2010)
Z. Ayati, J. Biazar, On the convergence of homotopy perturbation method. J. Egypt. Math. Soc. 23, 424–428 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Annex I: dataset
Annex I: dataset
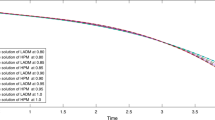
Table 1 shows, as explained in Eq. (67), the list of the maxima \(M_{1,k}=\max |u_{1,k}(\tau )|\), \(N_{1,k}=|v_{1,k}(\tau )|\), for \(\tau \in [0,6]\), corresponding to the first stage of case (2) (i.e. Sect. 5.2).
Table 2 shows the same data for the second stage of the same reaction, \(M_{2,k}=\max |u_{2,k}(\tau )|\), \(N_{2,k}=|v_{2,k}(\tau )|\), for \(\tau \in [1.55,6]\).
Rights and permissions
About this article
Cite this article
Bayón, L., Fortuny Ayuso, P., Grau, J.M. et al. Irreversible linear pathways in enzymatic reactions: analytical solution using the homotopy perturbation method. J Math Chem 58, 273–291 (2020). https://doi.org/10.1007/s10910-019-01080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-019-01080-7
Keywords
- Enzymatic kinetics
- Michaelis–Menten model
- Ordinary differential equations
- Homotopy perturbation method
- Optimization