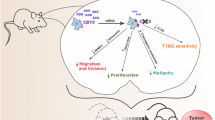
Abstract
Introduction
The orphan nuclear receptor 4A2 (NR4A2) has been extensively characterized in subcellular regions of the brain and is necessary for the function of dopaminergic neurons. The NR4A2 ligand, 1,1-bis (31-indoly1)-1-(p-chlorophenyl)methane (DIM-C-pPhCl) inhibits markers of neuroinflammation and degeneration in mouse models and in this study we investigated expression and function of NR4A2 in glioblastoma (GBM).
Methods
Established and patient-derived cell lines were used as models and the expression and functions of NR4A2 were determined by western blots and NR4A2 gene silencing by antisense oligonucleotides respectively. Effects of NR4A2 knockdown and DIM-C-pPhCl on cell growth, induction of apoptosis (Annexin V Staining) and migration/invasion (Boyden chamber and spheroid invasion assay) and transactivation of NR4A2-regulated reporter genes were determined. Tumor growth was investigated in athymic nude mice bearing U87-MG cells as xenografts.
Results
NR4A2 knockdown and DIM-C-pPhCl inhibited GBM cell and tumor growth, induced apoptosis and inhibited migration and invasion of GBM cells. DIM-C-pPhCl and related analogs also inhibited NR4A2-regulated transactivation (luciferase activity) confirming that DIM-C-pPhCl acts as an NR4A2 antagonist and blocks NR4A2-dependent pro-oncogenic responses in GBM.
Conclusion
We demonstrate for the first time that NR4A2 is pro-oncogenic in GBM and thus a potential druggable target for patients with tumors expressing this receptor. Moreover, our bis-indole-derived NR4A2 antagonists represent a novel class of anti-cancer agents with potential future clinical applications for treating GBM.
Graphic abstract








Similar content being viewed by others
References
Wansa KD et al (2002) The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem 277(36):33001–33011. https://doi.org/10.1074/jbc.M203572200
Giguere V (1999) Orphan nuclear receptors: from gene to function. Endocr Rev 20(5):689–725. https://doi.org/10.1210/Er.20.5.689
Wansa KD et al (2003) The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem 278(27):24776–24790. https://doi.org/10.1074/jbc.M300088200
Wang Z et al (2003) Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423(6939):555–560. https://doi.org/10.1038/nature01645
Maxwell MA, Muscat GE (2006) The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nuclear Recept Signal 4:e002. https://doi.org/10.1621/nrs.04002
Pearen MA, Muscat GE (2010) Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24(10):1891–1903. https://doi.org/10.1210/me.2010-0015
Mullican SE et al (2007) Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med 13(6):730–735. https://doi.org/10.1038/nm1579
Lee SO et al (2014) The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Mol Cancer Res 12(4):527–538. https://doi.org/10.1158/1541-7786.MCR-13-0567
Lee SO et al (2012) The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene 31(27):3265–3276. https://doi.org/10.1038/onc.2011.504
Wu H et al (2011) Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells. FASEB J 25(1):192–205. https://doi.org/10.1096/fj.10-166462
Zhou F et al (2014) Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat Commun 5:3388. https://doi.org/10.1038/ncomms4388
Safe S et al (2016) Nuclear receptor 4A (NR4A) family - orphans no more. J Steroid Biochem Mol Biol 157:48–60. https://doi.org/10.1016/j.jsbmb.2015.04.016
Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S (2015) Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocr Relat Cancer 22(5):831–840. https://doi.org/10.1530/ERC-15-0063
Hedrick E, Safe S (2017) Transforming growth factor beta/NR4A1-inducible breast cancer cell migration and epithelial-to-mesenchymal transition is p38alpha (mitogen-activated protein kinase 14) dependent. Mol Cell Biol 37(18):1. https://doi.org/10.1128/mcb.00306-17
Ke N et al (2004) Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res 64(22):8208–8212. https://doi.org/10.1158/0008-5472.CAN-04-2134
Sun L et al (2016) Notch signaling activation in cervical cancer cells induces cell growth arrest with the involvement of the nuclear receptor NR4A2. J Cancer 7(11):1388–1395. https://doi.org/10.7150/jca.15274
Komiya T et al (2010) Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene 29(11):1672–1680. https://doi.org/10.1038/onc.2009.453
Li X, Tai HH (2009) Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis 30(9):1606–1613. https://doi.org/10.1093/carcin/bgp161
Wang J et al (2013) Orphan nuclear receptor nurr1 as a potential novel marker for progression in human prostate cancer. Asian Pac J Cancer Prev 14(3):2023–2028
Llopis S et al (2013) Dichotomous roles for the orphan nuclear receptor NURR1 in breast cancer. BMC Cancer 13:139. https://doi.org/10.1186/1471-2407-13-139
Han Y et al (2013) Expression of orphan nuclear receptor NR4A2 in gastric cancer cells confers chemoresistance and predicts an unfavorable postoperative survival of gastric cancer patients with chemotherapy. Cancer 119(19):3436–3445. https://doi.org/10.1002/cncr.28228
Zhu B et al (2017) Activated Notch signaling augments cell growth in hepatocellular carcinoma via up-regulating the nuclear receptor NR4A2. Oncotarget 8(14):23289–23302. https://doi.org/10.18632/oncotarget.15576
Inamoto T et al (2008) 1,1-Bis(3′-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol Cancer Ther 7(12):3825–3833. https://doi.org/10.1158/1535-7163.MCT-08-0730
Han Y et al (2013) Nuclear orphan receptor NR4A2 confers chemoresistance and predicts unfavorable prognosis of colorectal carcinoma patients who received postoperative chemotherapy. Eur J Cancer 49(16):3420–3430. https://doi.org/10.1016/j.ejca.2013.06.001
Han YF, Cao GW (2012) Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol 18(47):6865–6873. https://doi.org/10.3748/wjg.v18.i47.6865
Beard JA et al (2015) The interplay of NR4A receptors and the oncogene-tumor suppressor networks in cancer. Cell Signal 27(2):257–266. https://doi.org/10.1016/j.cellsig.2014.11.009
Zhao BX et al (2006) p53 mediates the negative regulation of MDM2 by orphan receptor TR3. EMBO J 25(24):5703–5715. https://doi.org/10.1038/sj.emboj.7601435
Zhang T et al (2009) NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res 7(8):1408–1415. https://doi.org/10.1158/1541-7786.MCR-08-0533
Beard JA et al (2016) The orphan nuclear receptor NR4A2 is part of a p53-microRNA-34 network. Sci Rep 6:25108. https://doi.org/10.1038/srep25108
Zetterstrom RH et al (1996) Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res 41(1–2):111–120
Zetterstrom RH et al (1997) Dopamine neuron agenesis in Nurr1-deficient mice. Science 276(5310):248–250
Saucedo-Cardenas O et al (1998) Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA 95(7):4013–4018
Kadkhodaei B et al (2009) Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci 29(50):15923–15932. https://doi.org/10.1523/JNEUROSCI.3910-09.2009
Kadkhodaei B et al (2013) Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci USA 110(6):2360–2365. https://doi.org/10.1073/pnas.1221077110
Decressac M et al (2012) alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med 4(163):163ra156. https://doi.org/10.1126/scitranslmed.3004676
Decressac M et al (2013) NURR1 in Parkinson disease–from pathogenesis to therapeutic potential. Nat Rev Neurol 9(11):629–636. https://doi.org/10.1038/nrneurol.2013.209
Volakakis N et al (2015) Nurr1 and retinoid X receptor ligands stimulate ret signaling in dopamine neurons and can alleviate alpha-synuclein disrupted gene expression. J Neurosci 35(42):14370–14385. https://doi.org/10.1523/JNEUROSCI.1155-15.2015
De Miranda BR et al (2013) Neuroprotective efficacy and pharmacokinetic behavior of novel anti-inflammatory para-phenyl substituted diindolylmethanes in a mouse model of Parkinson’s disease. J Pharmacol Exp Ther 345(1):125–138. https://doi.org/10.1124/jpet.112.201558
De Miranda BR et al (2015) Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicol Sci 143(2):360–373. https://doi.org/10.1093/toxsci/kfu236
De Miranda BR et al (2015) The Nurr1 activator 1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane blocks inflammatory gene expression in BV-2 microglial cells by inhibiting nuclear factor kB. Mol Pharmacol 87(6):1021–1034. https://doi.org/10.1124/mol.114.095398
Hammond SL et al (2015) A novel synthetic activator of Nurr1 induces dopaminergic gene expression and protects against 6-hydroxydopamine neurotoxicity in vitro. Neurosci Lett 607:83–89. https://doi.org/10.1016/j.neulet.2015.09.015
Hammond SL et al (2018) The Nurr1 ligand,1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane, modulates glial reactivity and is neuroprotective in MPTP-induced parkinsonism. J Pharmacol Exp Ther. https://doi.org/10.1124/jpet.117.246389
Li X et al (2012) Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3′-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochem Pharmacol 83(10):1445–1455. https://doi.org/10.1016/j.bcp.2012.02.021
Chang LF et al (2011) Overexpression of the orphan receptor Nur77 and its translocation induced by PCH4 may inhibit malignant glioma cell growth and induce cell apoptosis. J Surg Oncol 103(5):442–450. https://doi.org/10.1002/jso.21809
Cancer Genome Atlas Research N et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372(26):2481–2498. https://doi.org/10.1056/nejmoa1402121
Brennan CW et al (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. https://doi.org/10.1016/j.cell.2013.09.034
Siegel RL et al (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Ostrom QT et al (2015) Epidemiology of gliomas. In: Raizer J, Parsa A (eds) Current understanding and treatment of gliomas, 1st edn. Springer, Switzerland, pp 1–14
Pearson JRD, Regad T (2017) Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther 2:17040. https://doi.org/10.1038/sigtrans.2017.40
Alexander BM, Cloughesy TF (2017) Adult Glioblastoma. J Clin Oncol 35(21):2402–2409. https://doi.org/10.1200/JCO.2017.73.0119
Acknowledgements
This work was supported by the National Institutes of Health [P30-ES023512 (SS), R01-ES025713 (SS), R01-CA202697 (SS), and T32-ES026568 (KK)], Texas A&M AgriLife Research (SS), the Sid Kyle Chair Endowment (SS), and the Karmanos Cancer Institute (SM).
Funding
This research received federal grant and University funding as indicated under funding.
Author information
Authors and Affiliations
Contributions
Study conception and design: S.K.M., S.M., S.H.S. Analysis and interpretation of data: All authors. Experimentation: K.K., S.K.M. Generation of Data: K.K., X.L., U.J., K.M., M.Z., S.K.M. Drafting of manuscript: S.K.M., S.M., S.H.S. Final approval of article: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karki, K., Li, X., Jin, UH. et al. Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas. J Neurooncol 146, 25–39 (2020). https://doi.org/10.1007/s11060-019-03349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03349-y