Abstract
Purpose
In Y90 radioembolization, the number of microspheres infused varies by more than a factor of 20 over the shelf-life of the glass radioembolization device. We investigated the effect of the number of Y90 microspheres on normal liver tissue.
Method
Healthy pigs received lobar radioembolization with glass Y90 microspheres at 4, 8, 12, and 16 days post-calibration, representing a > 20× range in the number of microspheres deposited per milliliter in tissue. Animals were survived for 1-month post-treatment and the livers were explanted and scanned on a micro CT system to fully characterize the microscopic distribution of individual microspheres. A complete 3D microdosimetric evaluation of each liver was performed with a spatially correlated analysis of histopathologic effect.
Results
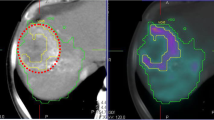
Through whole-lobe microscopic identification of each microsphere, a consistent number of microspheres per sphere cluster was found at 4, 8, and 12 days postcalibration, despite an 8-fold increase in total microspheres infused from days 4 to 12. The additional microspheres instead resulted in more clusters formed and, therefore, a more homogeneous microscopic absorbed dose. The increased absorbed-dose homogeneity resulted in a greater volume fraction of the liver receiving a potentially toxic absorbed dose based on radiobiologic models. Histopathologic findings in the animals support a possible increase in normal liver toxicity in later treatments with more spheres (i.e., ≥ day 12) compared to early treatments with less spheres (i.e., ≤ day 8).
Conclusion
The microdosimetric evidence presented supports a recommendation of caution when treating large volumes (e.g., right lobe) using glass 90Y microspheres at more than 8 days post-calibration, i.e., after “2nd week” Monday. The favorable normal tissue microscopic distribution and associated low toxicity of first week therapies may encourage opportunities for dose escalation with glass microspheres and could also be considered for patients with decreased hepatic reserve.






Similar content being viewed by others
Abbreviations
- TARE:
-
Trans-arterial radioembolization
- SA:
-
Specific activity
- NL:
-
Normal liver
- 90Y:
-
Yttrium-90
- MS/mL:
-
microspheres per milliliter
- DPK:
-
Dose-point kernel
- DVH:
-
Dose-volume histogram
- V20:
-
The percent volume of tissue receiving more than 20Gy
- CPE:
-
Charged particle equilibrium
- NTCP:
-
Normal tissue complication probability
- H&E:
-
Hematoxylin-eosin
- CVC:
-
Central-vein changes
- MIRD:
-
Medical internal radiation dose
- T:N:
-
Tumor to normal uptake ratio
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):393-424,
Fidelman N, Kerlan RK. Transarterial chemoembolization and 90y radioembolization for hepatocellular carcinoma: review of current applications beyond intermediate-stage disease. Am J Roentgenol. 2015;205(4):742-52.
Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 Radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155-63.
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, RTP P, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164-71
Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734-39.
Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71-81.
Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497-507.
Riaz A, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora R, et al. Radioembolization for hepatocellular carcinoma: statistical confirmation of improved survival in responders by landmark analyses. Hepatology. 2018;67:873–83 John Wiley & Sons, Ltd; [cited 2019 Jan 2]; Available from: http://doi.wiley.com/10.1002/hep.29480.
Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018;68:1429–40.
Ho CL, Chen S, Cheung SK, Leung YL, Cheng KC, Wong KN, et al. Radioembolization with 90Y glass microspheres for hepatocellular carcinoma: significance of pretreatment 11C-acetate and 18F-FDG PET/CT and posttreatment 90Y PET/CT in individualized dose prescription. Eur J Nucl Med Mol Imaging. 2018;45:2110–21.
Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: Review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87(1):22-32.
Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(3):1050-58.
Lewandowski RJ, Donahue L, Chokechanachaisakul A, Kulik L, Mouli S, Caicedo J, et al. (90) Y radiation lobectomy: outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol. 2016;114(1):99–105 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27103352.
Abecassis M, Caicedo JC, Memon K, Lewandowski RJ, Vouche M, Sato K, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013:1029–36 Available from: https://doi.org/10.1016/j.jhep.2013.06.015.
Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:94–100.
Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109-122.
Salem R, Padia SA, Lam M, Bell J, Chiesa C, Fowers K, et al. Clinical and dosimetric considerations for Y90 : recommendations from an International Multidisciplinary Working Group. Eur J Nucl Med Mol Imaging. 2019;46(8):1695-1704,
James T, Hill J, Fahrbach T, Collins Z. Differences in radiation activity between glass and resin 90y microspheres in treating unresectable hepatic cancer. Health Phys. 2017;112(3):300-304.
Walrand S, Hesse M, Chiesa C, Lhommel R, Jamar F. The low hepatic toxicity per gray of 90Y glass microspheres is linked to their transport in the arterial tree favoring a nonuniform trapping as observed in posttherapy PET imaging. J Nucl Med. 2014;55:135–40 Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.113.126839, http://www.ncbi.nlm.nih.gov/pubmed/24296766.
Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006;47:1209–11.
Elting F, Hasselager E, Friis C. Perfusion fixation of kidneys in adult pigs for electron microscopy. Cells Tissues Organs. 1977;98(3):340-42.
Simmons MM, Blamire IWH, Austin AR. Simple method for the perfusion-fixation of adult bovine brain. Res Vet Sci. 1996;60(3):247-50.
Latini F, Hjortberg M, Aldskogius H, Ryttlefors M. The use of a cerebral perfusion and immersion-fixation process for subsequent white matter dissection. J Neurosci Methods. 2015;253:161-69.
Crookston NR, Pasciak AS, Abiola G, Donnahue D, Weiss CR, Frey E. Verification of a method to detect glass microspheres via micro-CT. Med Phys. 2019. https://doi.org/10.1002/mp.13874. [Epub ahead of print].
Pasciak AS, Bourgeois AC, Bradley YC. A comparison of techniques for 90Y PET/CT image-based dosimetry following radioembolization with resin microspheres. Front Oncol. 2014;4:121 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid = 4033229&tool = pmcentrez&rendertype = abstract.
Pacilio M, Amato E, Lanconelli N, Basile C, Torres LA, Botta F, et al. Differences in 3D dose distributions due to calculation method of voxel S-values and the influence of image blurring in SPECT. Phys Med Biol. 2015;60:1945–64 Available from: http://iopscience.iop.org/article/10.1088/0031-9155/60/5/1945.
Eckerman K, Westfall R, Ryman J, Cristy M. Availability of nuclear decay data in electronic form, including beta spectra not previously published. Health Phys. 1994;67:338–45.
ICRU. Photon, electron, proton and neutron interaction data for body tissues. ICRU Rep. 1992;46.
Gulec SA, Sztejnberg ML, Siegel JA, Jevremovic T, Stabin M. Hepatic structural dosimetry in 90Y microsphere treatment: a monte carlo modeling approach based on lobular microanatomy. J Nucl Med. 2010;51(2):301-10.
Walrand S, Hesse M, Jamar F, Lhommel R. A hepatic dose-toxicity model opening the way toward individualized radioembolization planning. J Nucl Med. 2014;55:1317–22 Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.113.135301.
Attix FH. Charged-particle and radiation equilibria. Introd to Radiol Phys Radiat Dosim. 2007.
Jackson A, Ten Haken RK, Robertson JM, Kessler ML, Kutcher GJ, Lawrence TS. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. Int J Radiat Oncol Biol Phys. 1995;31(4):883-91.
Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp. Mol. Med. 2017;49(7):e359.
Lewandowski RJ, Minocha J, Memon K, Riaz A, Gates VL, Ryu RK, et al. Sustained safety and efficacy of extended-shelf-life 90Y glass microspheres: long-term follow-up in a 134-patient cohort. Eur J Nucl Med Mol Imaging. 2014;41(3):486-93.
Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56:339–46.
Fukuoka Y, Tanaka T, Nishiofuku H, Sato T, Masada T, Tatsumoto S, et al. Development of repeatable microcatheter access port for intra-arterial therapy of liver cancer. Cardiovasc Intervent Radiol. 2019.
Park UJ, Jeong W, Kwon SY, Kim Y, Choi K, Kim HT, et al. Fabrication of a novel absorbable vascular anastomosis device and testing in a pig liver transplantation model. Ann Biomed Eng. 2019;47(4):1063-77.
Pasciak AS, Bourgeois AC, Bradley YC. A microdosimetric analysis of tumor absorbed-dose as a function of the number of microspheres per unit volume in Yttrium-90 radioembolization. J Nucl Med. 2016;57(7):1020-26. https://doi.org/10.2967/jnumed.115.163444 Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.115.163444.
Acknowledgments
This project was supported by a grant from Biocompatibles UK Ltd, a BTG International group company. The investigators wish to acknowledge the contribution of Robert S. Balaban, Ph.D., and the National Heart, Lung and Blood Institute at the National Institute of Health for supplying the Micro CT system used in this study. We also wish to acknowledge input from Riad Salem, MD, in the writing of this manuscript.
Funding
This study was funded by Biocompatables, PLC
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author ASP, RPL, and CRW have received research grants from Biocompatables PLC. MRD is an employee of Biocompatables PLC. The remaining authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Digestive tract.
Electronic supplementary material
ESM 1
(DOCX 11325 kb).
Rights and permissions
About this article
Cite this article
Pasciak, A.S., Abiola, G., Liddell, R.P. et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur J Nucl Med Mol Imaging 47, 816–827 (2020). https://doi.org/10.1007/s00259-019-04588-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04588-x