Abstract
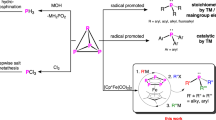
Phosphorus compounds are ubiquitous in the chemical sciences, finding applications throughout industry and academia. Of particular interest to synthetic chemists are organophosphorus compounds, which contain P–C bonds. However, state-of-the-art processes for the synthesis of these important materials rely on an inefficient, stepwise methodology involving an initial oxidation of white phosphorus (P4) with hazardous chlorine gas and the subsequent displacement of chloride ions. Catalytic P4 organofunctionalization reactions have remained elusive, as they require multiple P–P bond-breaking and P–C bond-forming events to break down the P4 core, all of which must occur in a controlled manner. Herein, we describe an efficient transition-metal-catalysed process capable of forming P–C bonds from P4. Using blue-light photocatalysis, this method directly affords valuable triarylphosphines and tetraarylphosphonium salts in a single reaction step.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the figures within the paper and other findings of this study are available from the corresponding author on reasonable request.
References
Corbridge, D. E. C. Phosphorus 2000. Chemistry, Biochemistry and Technology (Elsevier, 2000).
Schipper, W. Phosphorus: too big to fail. Eur. J. Inorg. Chem. 1567–1571 (2014).
Borger, J. E., Ehlers, A. W., Slootweg, J. C. & Lammertsma, K. Functionalization of P4 through direct P−C bond formation. Chem. Eur. J. 23, 11738–11746 (2017).
Wittig, G. & Schöllkopf, U. Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien (I. Mitteil.). Chem. Ber. 87, 1318–1330 (1954).
Wittig, G. & Haag, W. Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien (II. Mitteil.). Chem. Ber. 88, 1654–1666 (1955).
Guo, Y., Fu, H., Chen, H. & Li, X. Synthesis of new triarylphosphine ligand and their application in styrene hydroformylation. Catal. Commun. 9, 1842–1845 (2008).
Kamer, P. C. J., van Leeuwen, P. W. N. M. & Reek, J. N. H. Wide bite angle diphosphines: xantphos ligands in transition metal complexes and catalysis. Acc. Chem. Res. 34, 895–904 (2001).
Martin, R. & Buchwald, S. L. Palladium-catalyzed Suzuki−Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 41, 1461–1473 (2008).
Pignolet, L. M. (ed.) Homogeneous Catalysis with Metal Phosphine Complexes (Springer, 1983).
Surry, D. S. & Buchwald, S. L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 47, 6338–6361 (2008).
Fujihara, T., Yoshida, S., Terao, J. & Tsuji, Y. A triarylphosphine ligand bearing dodeca(ethylene glycol) chains: enhanced efficiency in the palladium-catalyzed Suzuki−Miyaura coupling reaction. Org. Lett. 11, 2121–2124 (2009).
Börner, A. & Franke, R. (eds) in Hydroformylation: Fundamentals, Processes, and Applications in Organic Synthesis 73–266 (Wiley, 2016).
El-Shahawi, M. S., Hassan, S. S. M., Othman, A. M., Zyada, M. A. & El-Sonbati, M. A. Chemical speciation of chromium(III,VI) employing extractive spectrophotometry and tetraphenylarsonium chloride or tetraphenylphosphonium bromide as ion-pair reagent. Anal. Chim. Acta 534, 319–326 (2005).
Starks, C. M. & Halper, M. Phase-Transfer Catalysis: Fundamentals, Applications, and Industrial Perspectives (Springer, 1994).
Kondo, S., Mori, T., Kunisada, H. & Yuki, Y. Synthesis of polymer-supported tetraphenylphosphonium bromides as effective phase-transfer catalysts at alkaline conditions. Makromol. Chem. Rapid Commun. 11, 309–313 (1990).
Manabe, K. Asymmetric phase-transfer alkylation catalyzed by a chiral quaternary phosphonium salt with a multiple hydrogen-bonding site. Tetrahedron Lett. 39, 5807–5810 (1998).
Ramanjaneyulu, B. T., Pareek, M., Reddy, V. & Vijaya Anand, R. Direct esterification of aromatic aldehydes with tetraphenylphosphonium bromide under oxidative N-heterocyclic carbene catalysis. Helv. Chim. Acta 97, 431–437 (2014).
Deng, Z., Lin, J.-H. & Xiao, J.-C. Nucleophilic arylation with tetraarylphosphonium salts. Nat. Commun. 7, 10337 (2016).
Reetz, M. T., Lohmer, G. & Schwickardi, R. A new catalyst system for the Heck reaction of unreactive aryl halides. Angew. Chem. Int. Ed. 37, 481–483 (1998).
Budnikova, Y. H., Gryaznova, T. V., Grinenko, V. V., Dudkina, Y. B. & Khrizanforov, M. N. Eco-efficient electrocatalytic C–P bond formation. Pure Appl. Chem. 89, 311–330 (2017).
Caporali, M., Gonsalvi, L., Rossin, A. & Peruzzini, M. P4 activation by late-transition metal complexes. Chem. Rev. 110, 4178–4235 (2010).
Scheer, M., Balázs, G. & Seitz, A. P4 activation by main group elements and compounds. Chem. Rev. 110, 4236–4256 (2010).
Khan, S., Sen, S. S. & Roesky, H. W. Activation of phosphorus by group 14 elements in low oxidation states. Chem. Commun. 48, 2169–2179 (2012).
Barton, D. H. R. & Zhu, J. Elemental white phosphorus as a radical trap: a new and general route to phosphonic acids. J. Am. Chem. Soc. 115, 2071–2072 (1993).
Barton, D. H. R. & Vonder Embse, R. A. The invention of radical reactions. Part 39. The reaction of white phosphorus with carbon-centered radicals. An improved procedure for the synthesis of phosphonic acids and further mechanistic insights. Tetrahedron 54, 12475–12496 (1998).
Cossairt, M. B. & Cummins, C. C. Radical synthesis of trialkyl, triaryl, trisilyl and tristannyl phosphines from P4. New J. Chem. 34, 1533–1536 (2010).
Ghosh, S. K., Cummins, C. C. & Gladysz, J. A. A direct route from white phosphorus and fluorous alkyl and aryl iodides to the corresponding trialkyl- and triarylphosphines. Org. Chem. Front. 5, 3421–3429 (2018).
König B. Chemical Photocatalysis (Walter de Gruyter, 2013).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 117, 10075–10166 (2016).
Marzo, L., Pagire, S. K., Reiser, O. & König, B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 57, 10034–10072 (2018).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Davies, A. G. in Chemistry of Tin (ed. Smith, P. J.) 265–289 (Springer, 1998).
Vyas, S. V., Lau, V. W. & Lotsch, B. V. Soft photocatalysis: organic polymers for solar fuel production. Chem. Mater. 28, 5191–5204 (2016).
Acknowledgements
We thank K. Zeitler (Universität Leipzig), O. Garcia Mancheño (Universität Münster) and J. C. Slootweg (University of Amsterdam) for valuable comments on the manuscript, P. Nitschke (Gschwind group, University of Regensburg) for assistance with NMR measurements and B. Luy (Karlsruhe Institute of Technology) for providing the broadband pulse xyBEBOP. Support by the DFG graduate program ‘Chemical Photocatalysis’ (GRK 1626) and the European Research Council (ERC CoG 772299) is also gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
U.L. developed the initial catalytic procedure. U.L. and P.B.A. performed further optimization. U.L., P.B.A. and D.J.S. investigated the substrate scope. U.L. and D.J.S. performed mechanistic investigations. V.S. and R.M.G. performed in situ NMR studies. C.R. performed (spectro)electrochemical experiments. R.W. oversaw and directed the project. U.L. and D.J.S. prepared the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, Figures 1–62, Tables 1–6 and references
Rights and permissions
About this article
Cite this article
Lennert, U., Arockiam, P.B., Streitferdt, V. et al. Direct catalytic transformation of white phosphorus into arylphosphines and phosphonium salts. Nat Catal 2, 1101–1106 (2019). https://doi.org/10.1038/s41929-019-0378-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0378-4
This article is cited by
-
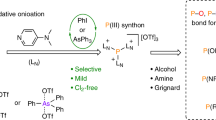
Direct conversion of white phosphorus to versatile phosphorus transfer reagents via oxidative onioation
Nature Chemistry (2022)
-
Phosphafluorenyl lithiums: direct synthesis from white phosphorus, structure and diversified synthons
Science China Chemistry (2022)
-
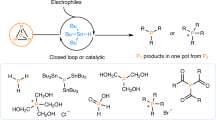
Synthesis of monophosphines directly from white phosphorus
Nature Chemistry (2021)
-
Pentaphosphaferrocene-mediated synthesis of asymmetric organo-phosphines starting from white phosphorus
Nature Communications (2021)