Abstract
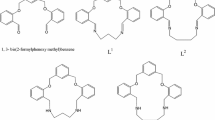
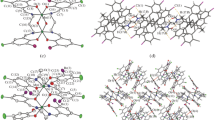
Two new phenol-based metal-free macrocyclic Schiff bases, cyclo-bis{2-[benz(N-propan-1,3-diyl)imidoyl][6-benzimidoyl][4-methyl]phenol} and cyclo-bis{2-[benz(N-butan-1,4-diyl)imidoyl][6-benzimidoyl][4-tert-butyl]phenol} have been synthesized and their structures determined by single crystal X-ray crystallography. The DFT geometry optimization calculations were performed to compare experimental and theoretical results. A comparison of the dihedral angles between mean planes of the central phenolato rings and peripheral phenyl rings in the crystal with the DFT theoretical calculations has been included for each molecule. Electronic transitions have been predicted by DFT molecular orbital calculations and compared with experimental absorption spectral data.
Graphic Abstract
A one-pot synthesis, crystal structure and theoretical calculations of 20- and 22-membered macrocyclic ligands are reported.










Similar content being viewed by others
References
Alexander V (1995) Chem Rev 95:273–342
Chu Z, Huang W, Wang L, Gou S (2008) Polyhedron 27:1079–1092
Tamburini S, Vigato PA (2004) Coord Chem Rev 248:1717–2128
Tian Y, Tong J, Frenzen G (1999) Sun J-Yu. J Org Chem 64:1442–1446
Atkins AJ, Black D, Blake AJ, Becerra AM, Parsons S, Ramirez LK, Schroder M (1996) Chem Commun 4:457–464
Tian YQ, Tong J (1997) Chin Chem Lett 8:107–110
Biwer A, Antranikian G, Heinzle E (2002) Appl Microbiol Biotechnol 59:609–617
Del Valle EMM (2010) Process Biochem 31:1033–1046
Thatiparti TR, Shoffstall AJ, Von Recum HA (2010) Biomaterials 31:2335–2347
Marcolino VA, Zanin GM, Durrant LR, Benassi MDT, Matioli GJ (2011) Agric Food Chem 59:3348–3357
De Oliveira V E, Almeida EWC, Castro HV, Edwards HGM, Dos Santos HF, de Oliveira LFC (2011) J Phys Chem A 115:8511–8519
Brusseau ML, Wang X (1997) Wang W -Z. Environ Sci Technol 31:1087–1092
Hoger S (2004) Chem Eur J 10:1320–1329
Zhang W, Moore JS (2005) J Am Chem Soc 127:11863–11870
Zang L, Che Y, Moore JS (2008) Acc Chem Res 41:1596–1608
Akine S, Taniguchi T, Nabeshima T (2001) Tetrahedron Lett 42:8861–8864
Gallant AJ, MacLachlan MJ (2003) Angew Chem Int Ed 42:5307–5310
Hui JK-H, MacLachlan MJ (2006) Chem Commun 23:2480–2482
Guieu S, Crane AK, MacLachlan MJ (2011) Chem Commun 47:1169–1171
Chen Z, Guieu S, White NG, Lelj F, MacLachlan MJ (2016) Chem Eur J 22:17657–17672
Pedersen CJ (1988) Angew Chem Int Ed Engl 27:1021–1027
Pedersen CJ (1967) J Am Chem Soc 86:7017–7036
Dietrich B, Lehn JM, Sauvage JP (1969) Tetrahedron Lett 10:2885–2888
Lehn J (1988) Angew Chem Int Ed Engl 27:89–112
Cram DJ, Kaneda T, Helgeson RC, Lein GM (1979) J Am Chem Soc 101:6752–6754
Cram DJ (1986) Angew Chem Int Ed Engl 25:1039–1134
Asatkar AK, Verma VK, Jain TA, Singh R, Gupta SK, Butcher RJ (2011) Acta Crystallogr E67:o2724–o2725
Gupta SK, Anjana C, Butcher RJ, Sen N (2010) Acta Crystallogr E66:m1531–m1532
Gupta SK, Anjana C, Sen N, Jasinski JP, Golen JA (2012) J Chem Crystallogr 42:960–967
Ganaie JA, Kumar J, Butcher RJ, Jasinski JP, Gupta SK (2016) J Chem Crystallogr 46:93–104
Gupta SK, Anjana C, Sen N, Butcher RJ, Jasinski JP, Golen JA (2015) Polyhedron 89:219–231
Gupta SK, Sen N, Ganaie JA, Butcher RJ, Jasinski JP (2017) J Coord Chem 70:3147–3170
Rigaku Oxford Diffraction (2015) CrysAlis Pro. The Woodlands
Sheldrick GM (2015) Acta Crystallogr A 71:3–8
Sheldrick GM (2015) Acta Crystallogr C 71:3–8
Sheldrick GM (2008) Acta Crystallogr A 64:112–118
Johnson CK (1976) ORTEP II. Report ORNL-5138. Oak Ridge National Laboratory, Oak Ridge
Allen FH, Kennard O, Watson DG, Brammer L, Orpen A, Taylor RJ (1987) Chem Soc Perkin Trans 2:S1–S19
Schmidt JR, Polik WF (2007) WebMO Pro, version 8.0.01e; WebMO, LLc: Holland. http://www.webmo.net
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Becke AD (1998) Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Hehre WJ, Random L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Pearl GM, Zerner MC, Broo A, McKelvey J (1998) J Comput Chem 19:781–796
Holland JP, Barnard PJ, Bayly SR, Dilworth JR, Green JC (2009) Inorg Chim Acta 362:402–406
Guillaumont D, Nakamura S (2000) Dyes Pigm 46:85–92
Origin 8.0. Origin Lab (2007) Origin Lab Corporation, Northampton
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555–1573
Acknowledgements
Financial support from the Government of India through the Department of Science and Technology [Project no. SR/S1/IC-38/2007] and the University Grants Commission [Project no. F.37-500/2009 (SR)] is gratefully acknowledged. RJB wishes to acknowledge the National Science Foundation for funds to purchase the diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ganaie, J.A., Sen, N., Butcher, R.J. et al. Synthesis, Crystal Structures, Density Functional Theory (DFT) Calculations and Molecular Orbital Calculations of Two New Metal-Free Macrocyclic Schiff Bases Derived from 2,6-Dibenzoyl-4-alkylphenol and Diamines. J Chem Crystallogr 50, 400–409 (2020). https://doi.org/10.1007/s10870-019-00812-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-019-00812-6