Abstract
The inverse association between physical activity and arterial thrombotic disease is well established. Evidence on the association between physical activity and venous thromboembolism (VTE) is divergent. We conducted a systematic review and meta-analysis of published observational prospective cohort studies evaluating the associations of physical activity with VTE risk. MEDLINE, Embase, Web of Science, and manual search of relevant bibliographies were systematically searched until 26 February 2019. Extracted relative risks (RRs) with 95% confidence intervals (CIs) for the maximum versus minimal amount of physical activity groups were pooled using random effects meta-analysis. Twelve articles based on 14 unique prospective cohort studies comprising of 1,286,295 participants and 23,753 VTE events were eligible. The pooled fully-adjusted RR (95% CI) of VTE comparing the most physically active versus the least physically active groups was 0.87 (0.79–0.95). In pooled analysis of 10 studies (288,043 participants and 7069 VTE events) that reported risk estimates not adjusted for body mass index (BMI), the RR (95% CI) of VTE was 0.81 (0.70–0.93). The associations did not vary by geographical location, age, sex, BMI, and methodological quality of studies. There was no evidence of publication bias among contributing studies. Pooled observational prospective cohort studies support an association between regular physical activity and low incidence of VTE. The relationship does not appear to be mediated or confounded by BMI.
Similar content being viewed by others
Introduction
Physical activity has several health benefits and its inverse and dose–response relationship with cardiovascular disease (CVD) (arterial thrombotic disease) is well established [1,2,3]. Physical activity may reduce vascular risk by exerting beneficial effects on physiological and metabolic processes through (1) improvements in endothelial function and levels of cardiovascular risk factors such as body weight, blood pressure, natriuretic peptides, lipid profiles, glucose tolerance, hemostatic factors, and cardiac troponin T [4,5,6]; (2) anti-inflammatory effects [7, 8]; and (3) enhancement of cardiac function [9, 10]. Venous thromboembolism (VTE) [comprising deep vein thrombosis (DVT) and pulmonary embolism (PE)], is closely linked with arterial thrombotic disease [11,12,13], and represents a growing public health burden due to increased morbidity, premature mortality, hospitalization, and associated healthcare costs [14,15,16,17]. The literature suggests both conditions may share some common risk factors such as obesity and cigarette smoking [18,19,20]. Factors implicated in the pathogenesis of VTE include inflammation, endothelial dysfunction, alterations in blood flow, immobilization, and hypercoagulable states [21,22,23] and physical activity is known to exert beneficial effects on some of these states [7, 24, 25]. Given the overall evidence, it is arguable that physical activity may lower the risk of VTE. Over the last decades, several studies have reported on the prospective associations of physical activity with the risk of VTE, but the results have been divergent. Some studies have reported decreased VTE risk with increased physical activity [26,27,28], whereas others have shown increased VTE risk with increased physical activity [29, 30] or no significant evidence of associations [31,32,33]. In a recent narrative review of existing observational prospective evidence, the authors evaluated and concluded that there may be a small beneficial effect of physical activity on the risk of incident VTE, but this did not appear to be consistent with a dose–response relationship [34]. A number of randomised controlled trials and observational studies have assessed the benefits and risks of physical activity on VTE, but these were based in patients or populations with acute or previous DVT [35]. A quantitative assessment of the association between physical activity and incident VTE using a systematic meta-analysis of the overall evidence has not yet been undertaken. Due to the wide uncertainty in the available evidence on this topic, we sought to evaluate in detail the prospective nature of the association between physical activity and future VTE risk using a systematic review and meta-analysis of all published observational prospective cohort studies conducted on the topic.
Methods
Data sources and searches
This systematic review and meta-analysis was registered in the PROSPERO prospective register of systematic reviews (CRD42019125869) and was conducted based on a predefined protocol and performed following PRISMA and MOOSE guidelines [36, 37] (Appendix 1–2 in ESM). We searched MEDLINE and Embase from inception to 26 February 2019. The computer-based searches used a combination of terms related to physical activity and VTE. There were no restrictions on language. Further details on the search strategy are presented in Appendix 3 in ESM. Titles and abstracts of studies retrieved from the databases were initially screened to assess their suitability for inclusion, after which we acquired potentially relevant articles for detailed full text evaluation. Two reviewers (SKK and SS) independently conducted full text evaluation using the inclusion criteria and any disagreements regarding eligibility of an article was discussed, and consensus reached with a third author (JAL). Reference lists of identified studies and relevant review articles were manually scanned and citing references were also checked in Web of Science, for additional eligible studies.
Study selection
Observational population-based prospective (cohort, case cohort, or nested case–control) studies were eligible for inclusion if they had at least 1 year of follow-up and examined the relation of regular physical activity with the risk of first VTE in adult general populations. Case–control study designs were not included.
Data extraction and quality assessment
One author (SKK) initially abstracted data from eligible studies using a standardized predesigned data collection form. A second reviewer (SS) independently checked these data with that in original articles. Any disagreements were discussed, and consensus reached with involvement of a third author (JAL). We extracted data on the following study characteristics: geographical location, period, design, participants (age, sex), sample size, duration of follow-up, assessment of physical activity, ascertainment of VTE case definition, number of participants developing VTE, and multivariate-adjusted relative risks (RRs), hazard ratios (HRs), or odds ratios (ORs) of VTE [and corresponding 95% confidence interval (CIs)]. Given emerging evidence that obesity (as measured using body mass index, BMI) is likely to reside in the causal pathway between physical activity and VTE, we also extracted data on the degree of adjustment for potential confounders (defined as ‘+’ minimally adjusted analysis, i.e. age and/or sex; ‘++’ as adjustment for established risk factors without BMI, i.e. age and/or sex plus cancer, socioeconomic status, smoking, or hypertension; and ‘+++’ as adjustment for established risk factors including BMI). To avoid double counting of a cohort, study selection was limited to a single set of most comprehensive results when there were multiple publications involving the same cohort. The priority for selection was the most up-to-date comprehensive study (longest follow-up or analysis covering the largest number of participants). We assessed the quality of studies on the basis of the nine-star Newcastle–Ottawa Scale (NOS) [38], which uses pre-defined criteria namely: selection (population representativeness), comparability (adjustment for confounders), and ascertainment of outcome. Nine points on the NOS reflects the highest study quality.
Data synthesis and analysis
Summary measures were presented as RRs with 95% CIs. Following Cornfield’s rare disease assumption [39], HRs and ORs were assumed to approximate the same measure of RR. The majority of studies divided subjects into 2 or more groups on the basis of occupational physical activity, leisure-time physical activity, intensity of physical activity, or total or any physical activity. To enable a consistent approach to the meta-analysis and enhance comparison and interpretation of the findings, the extreme groups (i.e. maximum vs minimal amount of physical activity) were used for the analyses. When the highest physical activity group was the referent, we converted the reported risk estimate into its reciprocal. When a risk estimate was reported as a continuous measure (e.g., per unit change), this was transformed to a top versus bottom quantile using standard statistical methods [40] described previously [41]. When a study assessed types of physical activity in addition to total or any physical activity, we only used risk estimates for total or any physical activity in the pooled analysis. When studies published more than one estimate of the association according to subgroups (e.g., by sex), we obtained a within-study summary estimate using a fixed effect meta-analysis. To minimize the effect of between-study heterogeneity, RRs were pooled using a random effects model [42]. Quantification of the extent of statistical heterogeneity across studies employed standard Chi square tests and the I2 statistic [43, 44]. The 95% prediction intervals were also estimated to determine the degree of heterogeneity, as they provide a region in which about 95% of the true effects of a new study are expected to be found [45, 46]. To ensure the robustness of our findings, we performed sensitivity analyses by omitting studies (one at a time) that could have influenced the pooled RR (e.g., study with the largest sample size, study that assessed physical activity exposure retrospectively, and study with participants recruited from a clinical trial) and calculated a pooled estimate for the remainder of the studies. Study-level characteristics including geographical location, sex, average age at baseline, average duration of follow-up, number of cases, type of VTE, degree of adjustment (with or without adjustment for BMI), and study quality were pre-specified as characteristics for assessment of heterogeneity, which was conducted using stratified analysis and random effects meta-regression [47]. To assess the potential for publication bias, we constructed and visually inspected Begg’s funnel plots [48] and performed Egger’s regression symmetry test [49]. All analyses were conducted using Stata version 15 (Stata Corp, College Station, Texas).
Results
Study identification and selection
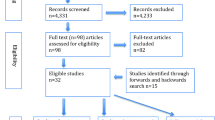
Figure 1 illustrates the study selection process. Our initial search and manual screening of citations identified 757 potentially relevant citations. After screening of titles and abstracts, 19 articles remained for full text evaluation. We reviewed and excluded 7 articles because (1) they duplicated a previous publication using the same cohort (n = 3); (2) they were review articles (n = 2); (3) was a case–control design (n = 1); and (4) exposure was not relevant (n = 1) In total, we included 12 articles [26,27,28,29,30, 33, 50,51,52,53,54,55] based on 14 unique cohort studies comprising of 1,286,295 participants and 23,753 VTE events.
Study characteristics and quality
Table 1 summarises characteristics of the 14 eligible studies on the association between physical activity and VTE. All 14 studies were based on prospective cohort study designs; however, one study was based on a prospective cohort follow-up of trial participants after the trial had been terminated [30]. For studies providing these data, the average age and BMI of participants at baseline ranged from approximately 46–65 years and 25.0–29.3 kg/m2 respectively; the weighted means were 52.1 years and 25.8 kg/m2 respectively. Four studies enrolled women only, two only men, and the rest enrolled both genders. Sample size of included cohorts ranged from 1766 to 992,228, with the number of VTE cases ranging from 171 to 14,550. The majority of studies (n = 9) included US populations, with five studies including European populations from Sweden, Norway, Denmark, and UK. Average duration of follow-up ranged from 5.0 to approximately 38.0 years, with a weighted mean of 9.6 years. All studies assessed physical activity through a questionnaire, but the categorisation of physical activity varied across studies. The diagnosis of VTE (DVT or PE) was based on a variety of methods which included duplex ultrasound, venogram, computed tomography, ventilation-perfusion scan, pulmonary angiogram, x-ray phlebography, and autopsy. Ascertainment of VTE was mostly based on self-reports, patient registries, hospital records, autopsy reports, or death registries. The degree of covariate adjustment varied, but majority of studies adjusted for risk factors such as age, sex, and BMI. Overall quality scores of studies ranged from 5 to 8.
Physical activity and incident VTE risk
The pooled fully-adjusted (including BMI) RR (95% CI) of VTE comparing the most physically active versus the least physically active groups was 0.87 (0.79–0.95) (Fig. 2). The 95% prediction interval for the pooled RR was 0.64–1.17%, suggesting that the true RR for any single new study will usually fall within this range. There was evidence of heterogeneity between the contributing studies (I2= 73%, 53–84%; p < 0.001), which was not explained by any of the study level characteristics prespecified for subgroup analysis (Fig. 3). On exclusion of the study which reported physical activity as a continuous measure and was initially designed as a clinical trial [30], the RR (95% CI) of VTE comparing the most physically active versus the least physically active groups was 0.84 (0.77–0.92), with evidence of heterogeneity between the contributing studies (I2= 69%, 45–82%; p < 0.001). On exclusion of the largest study which was made up of only women [28], the RR (95% CI) of VTE was 0.85 (0.75–0.95) with heterogeneity of (I2= 72%, 51–83%; p < 0.001). Furthermore, on exclusion of the study which assessed physical activity retrospectively [50], the RR (95% CI) of VTE was 0.88 (0.80–0.96) with heterogeneity of (I2= 72%, 51–84%; p < 0.001).
Prospective studies of physical activity and risk of venous thromboembolism included in meta-analysis. The summary estimate presented was calculated using random effects models and was based on fully adjusted estimates (including body mass index) where relevant; sizes of data markers are proportional to the inverse of the variance of the relative ratio; CI, confidence interval (bars); PA, physical activity; RR, relative risk; VTE, venous thromboembolism; study abbreviations are listed in Table 1
Relative risks for venous thromboembolism comparing maximal versus minimal amount of physical activity, grouped according to several study characteristics. The summary estimates presented were calculated using random effects models; CI, confidence interval (bars); PA, physical activity; RR, relative risk; VTE, venous thromboembolism; *, p value for meta-regression; **, defined as ‘+’ minimally adjusted analysis (age and/or sex); ‘++’ as adjustment for established risk factors without body mass index (age and/or sex plus cancer, socioeconomic status, smoking, or hypertension); and ‘+++’ as adjustment for established risk factors including body mass index; †, number of cases and participants are not equal across all the subgroups because not all studies reported data on these study characteristics
In pooled analysis of 10 studies (288,043 participants and 7069 VTE events) that reported risk estimates not adjusted for BMI, the RR (95% CI) of VTE was 0.81 (0.70–0.93) (Appendix 4 in ESM).
Publication bias
A funnel plot of the 14 studies reporting on the associations between physical activity and VTE risk showed visual evidence of symmetry (Appendix 5 in ESM) which was consistent with Egger’s regression symmetry test (p = 0.065). We also found no evidence of such selective reporting when studies were grouped by size in meta-regression analysis (Fig. 3).
Comments
Summary of main findings
Given the uncertain evidence on the prospective relationship between physical activity and VTE risk as well as the absence of any quantitative evidence summarizing the existing literature, we have conducted the first meta-analysis of population-based prospective cohort studies to evaluate the association between regular physical activity and VTE. In pooled analysis of 14 studies, regular physical activity was significantly associated with a lower risk of VTE when compared with a sedentary or less active lifestyle. In contrast to previous studies, the association did not appear to be mediated or confounded by BMI. Furthermore, the inverse association between physical activity and VTE risk was not modified by geographical location, sex, age, duration of follow-up, degree of adjustment, and methodological quality of studies.
Comparison with previous work
To the best of our knowledge, there have been no previous efforts to aggregate existing data on the relation between physical activity and future VTE risk quantitatively. Being the first comprehensive meta-analysis on the topic, the findings cannot be directly compared to previous work. Though there has been overwhelming evidence showing that regular physical activity is associated with reduced risk of arterial thrombotic disease [1,2,3], findings on the relationship between physical activity and VTE risk have been mixed in the absence of a pooled analysis. In a recently published narrative review that evaluated the existing epidemiological evidence on the association between physical activity and VTE risk, Evensen and colleagues concluded that there might be a modest beneficial effect of physical activity on incident VTE risk [34]. However, the evidence was hampered by the variance in the assessment and definition of the exposure variable—physical activity. By combining the available evidence in a systematic and quantitative manner, we have shown that regular physical activity is also associated with a reduced risk of VTE compared with physical inactivity. In contrast to evidence from some individual studies [26, 53], the association did not appear to be mediated or confounded by BMI. Though there was no statistically significant evidence of effect modification by age on the association, there was a suggestion that the beneficial effect of physical activity on VTE risk was stronger in the elderly, a finding which is consistent with previous evidence [53].
Possible explanations for findings
To date, obesity and smoking have consistently been demonstrated to be associated with VTE risk [18,19,20, 26]. The current findings suggest that another common lifestyle factor shared by VTE and atherosclerotic CVD could be physical inactivity. Both disease states share common characteristics such as coagulation and platelet activation, hence they may have common pathophysiological mechanisms [56]. On the contrary, atherosclerotic CVD and VTE have historically been viewed as two distinct diseases [57] and based on findings that traditional risk factors for VTE and arterial thrombotic disease are not similar, it is generally believed that their pathogenesis differ [31, 58]. The pathophysiological mechanisms underlying the association between regular physical activity and reduced VTE risk may relate to the ability of physical activity to (1) improve levels of potential risk factors such as body weight, hypertension, and lipids; [59] (2) decrease systemic inflammation; [7, 8] and (3) decrease plasma viscosity [25] and platelet aggregation [60], all of which are involved in VTE pathophysiology. Increased muscular activity of the lower limbs as a result of regular physical activity could also increase venous return and decrease VTE risk [61]. Mechanistic conclusions underlying the association between physical activity and VTE cannot be drawn from observational epidemiological studies and need further specific studies to clarify these pathways.
Implications of findings
The potential association between regular physical activity and decreased VTE risk may have clinical implications with respect to VTE prevention. Just like CVD, physical activity may represent an important approach for VTE prevention, for instance in the areas of screening of individuals at risk of VTE, recommending lifestyle modification, as well as further management. Though there is no clinical trial evidence showing regular physical activity can reduce the incidence of VTE, RCT evidence shows physical activity to be associated with reduced severity of VTE complications such as post thrombotic syndrome [35]. The health benefits associated with regular PA, cannot be overemphasized. The Physical Activity Guidelines Advisory Committee Scientific Report recommends 150–300 min/week of moderate-intensity or 75–150 min/week of vigorous-intensity aerobic PA/exercise for adults, as this is associated with substantial health benefits in most people; [62] however, a substantial proportion of the population do not achieve these recommended levels. For example in the United States, only 46% of adults meet the general physical activity recommendations [63]. Though there are still some unanswered questions such as the nature of the dose–response relationship between physical activity and VTE risk and the optimal physical activity intensity, frequency, and duration for VTE prevention, it is recommended that physically inactive adults should engage in some regular physical activity to improve their overall vascular health. Even standing, which is the least active behaviour, has been reported to be associated with health benefits compared with sitting [64].
Strengths and limitations
Given the inconsistent evidence on the topic, this study represents the first attempt at summarising the overall evidence using a systematic meta-analysis. We employed a comprehensive search strategy across multiple databases with no language restrictions and undertook manual reference scanning; which made it unlikely that we had missed any relevant study conducted on the topic. Other strengths were the comprehensive analyses which included exploration of heterogeneity using stratification by several study level characteristics and several sensitivity analyses. Finally, formal tests showed no evidence of publication bias or selective reporting. However, despite the lack of strong evidence of publication bias, we cannot completely rule out the influence of selective reporting; since tests for publication bias have low statistical power. There were important limitations to this review and these were all inherent to the included studies. The included studies examined different types of physical activity and categorised the amount of physical activity differently, hence comparisons could only be made between the most and least active and there was difficulty in combining data across studies to assess a dose–response relation between physical activity and VTE. The effect of the different types/modalities of physical activity could not be explored because of the limited number of studies consistently reporting on the same type of physical activity. There was the potential for misclassification bias because physical activity was self-reported and its classification was study-specific. Studies did not report risk estimates for the specific endpoints of DVT and PE and therefore their associations could not be evaluated. There was substantial heterogeneity between contributing studies which could not be explained by several clinically relevant study level characteristics, suggesting that other factors might be at play. One study based on only women contributed about 77% of the overall sample size, however, exclusion of this study in sensitivity analysis did not change the overall estimate. Pooled analysis was based on variably adjusted data reported by the eligible studies, therefore prone to confounding by unmeasured factors. Finally, estimated prediction intervals of the pooled RRs of the associations contained values on both sides of the null and so, although on average there was evidence of an association of regular physical activity with VTE risk, this may not always be the case in other studies. The findings should therefore be interpreted with caution given these limitations. To address the issues with standardization of physical activity, consistent adjustment for confounding, exploration of dose–response relationships and assessment of heterogeneity, we propose an individual participant data meta-analysis of these prospective cohort studies.
Conclusion
New evidence based on a comprehensive meta-analysis of all observational prospective cohort studies support an association between regular physical activity and low incidence of VTE. In contrast to previous evidence, the potential protective effect of physical activity on VTE does not appear to be mediated or confounded by BMI. These findings should stimulate further efforts to further clarify the relationship between physical activity and VTE risk.
References
Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol. 2018;25(17):1864–72. https://doi.org/10.1177/2047487318795194.
Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54. https://doi.org/10.1016/S0140-6736(17)31634-3.
Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. https://doi.org/10.1136/bmj.i3857.
Tran ZV, Weltman A, Glass GV, Mood DP. The effects of exercise on blood lipids and lipoproteins: a meta-analysis of studies. Med Sci Sports Exerc. 1983;15(5):393–402.
Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92. https://doi.org/10.1056/NEJMoa020194.
Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412–9. https://doi.org/10.1001/jama.295.12.1412.
Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13(5):561–8. https://doi.org/10.1097/01.ede.0000023965.92535.c0.
Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22(11):1869–76.
Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342(7):454–60. https://doi.org/10.1056/NEJM200002173420702.
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. https://doi.org/10.1056/NEJMoa011858.
Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348(15):1435–41. https://doi.org/10.1056/NEJMoa022157.
Sorensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370(9601):1773–9. https://doi.org/10.1016/S0140-6736(07)61745-0.
Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost JTH. 2008;6(11):1851–7. https://doi.org/10.1111/j.1538-7836.2008.03102.x.
Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–64.
Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–69. https://doi.org/10.1093/eurheartj/ehu283.
Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–7. https://doi.org/10.1161/CIRCRESAHA.115.306841.
Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291–302. https://doi.org/10.1160/TH12-03-0162.
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. https://doi.org/10.1161/CIRCULATIONAHA.107.709204.
Mahmoodi BK, Cushman M, Anne Naess I, et al. Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation. 2017;135(1):7–16. https://doi.org/10.1161/CIRCULATIONAHA.116.024507.
Gregson J, Kaptoge S, Bolton T, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 2019;4(2):163–73. https://doi.org/10.1001/jamacardio.2018.4537.
Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365(9465):1163–74. https://doi.org/10.1016/S0140-6736(05)71880-8.
van Adrichem RA, Debeij J, Nelissen RG, Schipper IB, Rosendaal FR, Cannegieter SC. Below-knee cast immobilization and the risk of venous thrombosis: results from a large population-based case-control study. J Thromb Haemost JTH. 2014;12(9):1461–9. https://doi.org/10.1111/jth.12655.
Healy B, Levin E, Perrin K, Weatherall M, Beasley R. Prolonged work- and computer-related seated immobility and risk of venous thromboembolism. J R Soc Med. 2010;103(11):447–54. https://doi.org/10.1258/jrsm.2010.100155.
Sherman DL. Exercise and endothelial function. Coron Artery Dis. 2000;11(2):117–22.
Folsom AR, Wu KK, Davis CE, Conlan MG, Sorlie PD, Szklo M. Population correlates of plasma fibrinogen and factor VII, putative cardiovascular risk factors. Atherosclerosis. 1991;91(3):191–205. https://doi.org/10.1016/0021-9150(91)90167-2.
Wattanakit K, Lutsey PL, Bell EJ, et al. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost. 2012;108(3):508–15. https://doi.org/10.1160/TH11-10-0726.
Olson NC, Cushman M, Judd SE, et al. American Heart Association’s Life’s Simple 7 and risk of venous thromboembolism: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2015;4(3):e001494. https://doi.org/10.1161/JAHA.114.001494.
Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ, Million Women Study C. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131(8):721–9. https://doi.org/10.1161/circulationaha.114.010296.
van Stralen KJ, Doggen CJ, Lumley T, et al. The relationship between exercise and risk of venous thrombosis in elderly people. J Am Geriatr Soc. 2008;56(3):517–22. https://doi.org/10.1111/j.1532-5415.2007.01588.x.
Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–82. https://doi.org/10.1093/aje/kwi309.
Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–9.
Kabrhel C, Varraso R, Goldhaber SZ, Rimm E, Camargo CA Jr. Physical inactivity and idiopathic pulmonary embolism in women: prospective study. BMJ. 2011;343:d3867. https://doi.org/10.1136/bmj.d3867.
Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121(17):1896–903. https://doi.org/10.1161/CIRCULATIONAHA.109.921460.
Evensen LH, Braekkan SK, Hansen JB. Regular physical activity and risk of venous thromboembolism. Semin Thromb Hemost. 2018;44(8):765–79. https://doi.org/10.1055/s-0038-1673636.
Kahn SR, Shrier I, Kearon C. Physical activity in patients with deep venous thrombosis: a systematic review. Thromb Res. 2008;122(6):763–73. https://doi.org/10.1016/j.thromres.2007.10.011.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology. JAMA J Am Med Assoc. 2000;83(15):2008–12. https://doi.org/10.1001/jama.283.15.2008.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 20 August.
Cornfield J. A method of estimating comparative rates from clinical data; applications to cancer of the lung, breast, and cervix. J Natl Cancer Inst. 1951;11(6):1269–75.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9.
Kunutsor SK, Apekey TA, Cheung BM. Gamma-glutamyltransferase and risk of hypertension: a systematic review and dose-response meta-analysis of prospective evidence. J Hypertens. 2015;33(12):2373–81. https://doi.org/10.1097/HJH.0000000000000763.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. https://doi.org/10.1136/bmj.d549.
Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–59. https://doi.org/10.1111/j.1467-985X.2008.00552.x.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708. https://doi.org/10.1002/(sici)1097-0258(19991030)18:20%3c2693:aid-sim235%3e3.0.co;2-v.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Lindqvist PG, Epstein E, Olsson H. The relationship between lifestyle factors and venous thromboembolism among women: a report from the MISS study. Br J Haematol. 2009;144(2):234–40. https://doi.org/10.1111/j.1365-2141.2008.07460.x.
Lutsey PL, Virnig BA, Durham SB, et al. Correlates and consequences of venous thromboembolism: the Iowa Women’s Health Study. Am J Public Health. 2010;100(8):1506–13. https://doi.org/10.2105/AJPH.2008.157776.
Ogunmoroti O, Allen NB, Cushman M, et al. Association between life’s simple 7 and noncardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2016. https://doi.org/10.1161/jaha.116.003954.
Evensen LH, Isaksen T, Hindberg K, Braekkan SK, Hansen JB. Repeated assessments of physical activity and risk of incident venous thromboembolism. J Thromb Haemost JTH. 2018;16(11):2208–17. https://doi.org/10.1111/jth.14287.
Kim J, Kraft P, Hagan KA, Harrington LB, Lindstroem S, Kabrhel C. Interaction of a genetic risk score with physical activity, physical inactivity, and body mass index in relation to venous thromboembolism risk. Genet Epidemiol. 2018;42(4):354–65. https://doi.org/10.1002/gepi.22118.
Johansson M, Johansson L, Wennberg P, Lind M. Physical activity and risk of first-time venous thromboembolism. Eur J Prev Cardiol. 2019. https://doi.org/10.1177/2047487319829310.
Ray JG. Dyslipidemia, statins, and venous thromboembolism: a potential risk factor and a potential treatment. Curr Opin Pulm Med. 2003;9(5):378–84.
Prandoni P. Venous thromboembolism and atherosclerosis: is there a link? J Thromb Haemost JTH. 2007;5(Suppl 1):270–5. https://doi.org/10.1111/j.1538-7836.2007.02467.x.
Smabrekke B, Rinde LB, Hindberg K, et al. Atherosclerotic risk factors and risk of myocardial infarction and venous thromboembolism; time-fixed versus time-varying analyses. The Tromso Study. PLoS ONE. 2016;11(9):e0163242. https://doi.org/10.1371/journal.pone.0163242.
Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2007;14(1):12–7. https://doi.org/10.1097/HJR.0b013e3280128bbb.
Heber S, Volf I. Effects of physical (in)activity on platelet function. Biomed Res Int. 2015;2015:165078. https://doi.org/10.1155/2015/165078.
Sochart DH, Hardinge K. The relationship of foot and ankle movements to venous return in the lower limb. J Bone Joint Surg Br. 1999;81(4):700–4.
Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8. https://doi.org/10.1001/jama.2018.14854.
Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: united States, 2008–2010. Vital Health Stat. 2013;10(257):1–184.
van der Ploeg HP, Chey T, Ding D, Chau JY, Stamatakis E, Bauman AE. Standing time and all-cause mortality in a large cohort of Australian adults. Prev Med. 2014;69:187–91. https://doi.org/10.1016/j.ypmed.2014.10.004.
Funding
This study was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol (BRC-1215-20011). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. THM and SKK acknowledge support from the Division of Cardiology, Department of Internal Medicine, Oulu University Hospital, Oulu, Finland via the Finnish Governmental Research Funding (VTR). SS acknowledges support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care – East Midlands (NIHR CLAHRC – EM), the Leicester Clinical Trials Unit and the NIHR Leicester-Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University and the University of Leicester.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kunutsor, S.K., Mäkikallio, T.H., Seidu, S. et al. Physical activity and risk of venous thromboembolism: systematic review and meta-analysis of prospective cohort studies. Eur J Epidemiol 35, 431–442 (2020). https://doi.org/10.1007/s10654-019-00579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-019-00579-2