Abstract
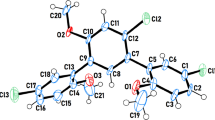
In the solid state, some 3-substituted 4-hydroxycoumarins β-ketoester enols form infinite translational hydrogen-bonded β-chains with varying degrees of alignment between adjacent delocalized systems. Nine related structures have been studied. At the strongest, intermolecular associations are polar, purely translation neighbors interact essentially along a 717 pm crystallographic repeat with shortened 260 pm intermolecular O·OH-bond contacts. Four distinctive features characterize these structures: (1) moderately delocalized β-ketoester enol structures, (2) translational misalignment angles between oxygen donors and acceptors less than 10°, (3) buttressing intermolecular C–H·O contacts co-planar with and near the intermolecular O–H·O interactions, and (4) fully extended ketoester enol hydrogen-bond (ap-anti-anti) geometries. For non-polar β-chains in related coumarin systems, β-ketoester enol alignments are typically poorer, involve hydrogen-bonding between glide relatives, ap-syn-(anti) geometry, and the intermolecular O·OH-bond contacts are longer.
Graphic Abstract
Substituted 4-hydroxycoumarins related to phenprocoumon can form well-aligned polar translational β-chains between enolones showing resonance assisted Hydrogen-bonding and a 717 pm repeat along a crystallographic axis.







Similar content being viewed by others
References
Steed JW, Turner DR, Wallace KJ (2007) Core concepts in supramolecular chemistry and nanochemistry. Wiley, Chichester
Gilli G, Bellucci F, Ferretti V, Bertolasi V (1989) Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the β-diketone fragment. J Am Chem Soc 111:1023–1028
Bertolasi V, Gilli P, Ferretti V, Gilli G (1996) Resonance-assisted O-H…O hydrogen bonding: its role in the crystalline self-recognition of β-diketone enols and its structural and IR characterization. Chem Eur J 2(8):925–934
Sanz P, Mo O, Yanez M, Elguero J (2007) Resonance-assisted hydrogen bonds: a critical examination. structure and stability of the enols of β-diketones and β-enaminones. J Phys Chem 111:3585–3591
Gora RW, Maj M, Grabowski SJ (2013) Resonance-assisted hydrogen bonds revisited Resonance stabilization vs. charge delocalization. Phys Chem Chem Phys 15:2514–2522
Gilli P, Pretto L, Bertolasi V, Gilli G (2009) Predicting hydrogen-bond strengths from acid-base molecular properties the pKa slide rule: toward the solution of a long-lasting problem. Acc Chem Res 42(1):33–44
Tadeusz M, Krygowski J, Zachara-Horeglad E (2009) Resonance-assisted hydrogen bonding in terms of substituent effect. Tetrahedron 65:2010–2014
Beck JF, Mo Y (2007) How resonance assists hydrogen bonding interactions: an energy decomposition analysis. J Comput Chem 28:455–466
Grabowski JS (2009) Covalent character of hydrogen bonds enhanced by π-electron delocalization. Croat Chem Acta 82:185–192
Gilli G Bertolasi, Feretti V, Gilli P (1993) Resonance assisted hydrogen bond. III. Formation of intermolecular hydrogen-bonded chains in crystals of β-diketones and its relevance to molecular association. Acta Crystallogr B49:564–576
Trifonov L, Bieri JH, Prewo R, Dreiding AS, Rast DM, Hoesch L (1982) The constitution of veretinolide, a new derivative of tetronic acid, Produced by Verticillium Intertextum. Tetrahedron 38(3):397–403
Katrusiak A (1993) Structure of 2-methyl-1,3-cyclohexandione crystals. J Crystallogr Spectrosc Res 23(5):367–372
Gaultier J, Hauw C (1966) Structure del’hydroxy-4-coumarine. Eau d’Hydroation et Cohesion Cristalline. Acta Crystallogr 20:646–651
Bravic G, Gaultier J, Hauw C (1971) Structure cristalline et moleculaire du marcoumar. C R Acad Sci Paris C272:1112–1114
Bravic G, Gaultier J, Geoffre S, Hauw C (1974) Structure crystalline d’une antivitamine K: 1’α-naphthyl-3-hydroxy-4-coumarine. C R Acad Sci Paris 278:601–603
Valente EJ, Trager WF, Lingafelter EC (1976) (–)–3–(1–Phenylpropyl)–4–hydroxycoumarin. Acta Crystallogr B32:277–279
Manolov I, Maichle-Moessmer C (2007) Crystal structure of 4-hydroxy-3-[1-phenyl-2-(4-methoxybenzoyl)ethyl]-2H-1-benzopyran-2-one. Anal Sci 23:X79 (See CCDC 637813)
Bravic G, Gaultier J, Hauw C (1968) Structure crystalline et moleculaire du dicoumarol. C R Acad Sci Paris 267:1790–1795
Alcock NW, Hough E (1972) The crystals and molecular structure of 3,3-methylene(bis-6-bromo-4-hydroxycoumarin): unusual molecular interactions. Acta Crystallogr B28:1957–1960
Valente EJ, Eggleston DS (1989) Structure of (phenyl)bis(4-hydroxybenzo-2H-pyran-2-one-3-yl)methane. Acta Crystallogr C45:785–787
Stancheva S, Maichle-Mössmerb C, Manolova I (2007) Synthesis, structure and acid-base behaviour of some 4-hydroxycoumarin derivatives. Zeitschrift fur Naturforschung 62:737–741
Ravikumar N, Gopikrishna G, Solomon KA (2012) 3,3’-[(4-Nitrophenyl)methylene]-bis(4-hydroxy-2H-chromen-2-one). Acta Crystallogr E 68:o265
Li M-K, Li J, Liu B-H, Zhou Y, Li X, Xue X-Y, Hou Z, Luo X-X (2013) Synthesis, crystal structures, and anti-drug-resistant Staphylococcus aureus activities of novel 4-hydroxycoumarin derivatives. Eur J Pharmacol 721:151–157
Chang-Wei LV, Yao-Ping WU, Jian L, Xin-Gang J (2012) 3,3’-(4-Dimethylamino benzylidene)-bis-(4-hydroxycoumarin). Chin J Struct Chem 31:847–850
Manolol I, Ströebele M, Meyer H-J (2008) Crystal structure of 4-hydroxy-3-[(2-oxo-2H-chromen-3-yl)-(3,4,5-trimethoxyphenyl)methyl]chromen-2-one. Anal Sci 24:x135–x136
Manolov I, Morgenstern B, Hegetschweiler K (2012) Synthesis and structure of ethyl 2-[bis(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]benzoate. X-Ray Struct Anal Online 28:83–84
Manolov I, Morgenstern B, Hegetschweiler K (2012) Synthesis and Structure of 4-Hydroxy-3-[(2-oxo-2H-chromen-3-yl)-3,4-dihydroxyphenyl)methyl]chromen-2-one ethanol solvate. X-Ray Struct Anal Online 28:87–88
Manolov I, Maichle-Moessmer C (2013) Synthesis and crystal structure of 4-hydroxy-3-[(3E)-3-(hydroxyimino)-1-(4-nitrophenyl)butyl]-2H-chromen-2-one. Bulg Chem Commun 45(1):109–113
Asas M, Oo C-W, Osman H, Quah CK, Fun H-K (2010) 3-[(E)-3-(2,4-Dichloro phenyl)prop-2-enoyl]-4-hydroxy-2H-chromen-2-one. Acta Crystallogr 66E:o3022–o3023
Naveen S, Adlakha P, Upadhyay K, Shah A, Anandalwar SM, Prasad JS (2006) Crystal structure of 3-nitro-4-hydroxycoumarin. Anal Sci 22:x103–x104
Stefanou V, Matiadis D, Melagraki G, Afantitis A, Athanasellis G, Igglessi-Markopoulou O, McKee V, Markopoulos J (2011) Functionalized 4-hydroxycoumarins: novel synthesis, crystal structure and DFT calculations. Molecules 16:384–402
Pohl LR, Haddock R, Garland WA, Trager WF (1975) Synthesis and thin-layer chromatographic, ultraviolet, and mass spectral properties of the anticoagulant phenprocoumon and its monohydroxylated derivatives. J Med Chem 18(5):513–519
West BD, Link KP (1965) The resolution and absolute configuration of marcumar. J Heterocycl Chem 2(1):93–94
Kischel J, Mertins K, Michalik D, Zapf A, Bellera M (2007) A general and efficient iron-catalyzed benzylation of 1,3-dicarbonyl compounds. Adv Synth Catal 2007(349):865–870
Theerthagiri P, Lalitha A (2010) Benzylation of β-dicarbonyl compounds and 4-hydroxycoumarin using TMSOTf catalyst: a simple, mild, and efficient method. Tetrahedron 51:5454–5458
West BD, Preis S, Schroeder CH, Link KP (1961) Studies on 4-hydroxycoumarins. XVII. The resolution and absolute configuration of warfarin. J Am Chem Soc 83(12):2676–2679
Liu Z-Q, Zhang Y, Zhao L, Li Z, Wang J, Li H, Wu L-M (2011) Iron-Ctalyzed stereospecific olefin synthesis by direct coupling of alcohols and alkenes with alcohols. Org Lett 13(9):2208–2211
Appendino G, Cravotto G, Tagliapietra S, Ferraro S, Nano GM (1991) The chemistry of coumarin derivatives. Part 3. Synthesis of 3-alkyl-4-hydroxycoumarins by reductive fragmentation of 3,3’-alkylidine-4,4’-dihydroxybis[coumarins]. Helv Chim Acta 74:1451–1458
Stahmann MA, Wolff I, Link KP (1943) Studies on 4-hydroxycoumarins. I. The synthesis of 4-hydroxycoumarins. J Am Chem Soc 65:2285–2287
Clark RS, Reid JS (1995) The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr A51:887–897
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122 (recent program release from 2018)
Mercury (2016) version 3.9, Cambridge Crystallographic Data Center
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76
Valente EJ, Eggleston DS, Schomaker V (1986) Structures of Five trans–2–hydroxy and methoxy–2–methyl–3,4–dihydro–4-aryl–2H,5H–pyrano [3,2–c] [1]benzopyran–5–ones. Acta Crystallogr A C42:1809–1813
Etter MC, MacDonald JC, Bernstein J (1990) Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr A B46:256–262
Acknowledgements
EJV thanks the National Science Foundation (MRI-0618148) for support of crystallographic equipment. Thanks also go to Dr. Verner Schomaker (deceased) of the University of Washington for his encouragement, acumen, persistence and pedagogy with difficult structures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duong, TV.H., Carroll, T.S., Bejan, D.S. et al. β-Chain Hydrogen-Bonding in 4-Hydroxycoumarins. J Chem Crystallogr 50, 387–399 (2020). https://doi.org/10.1007/s10870-019-00813-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-019-00813-5