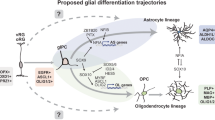
Abstract
The establishment of neuronal and glial networks in the brain depends on the activities of neural progenitors, which are influenced by cell-intrinsic mechanisms, interactions with the local microenvironment and long-range signaling. Progress in neuroscience has helped identify key factors in CNS development. In parallel, studies in recent years have increased our understanding of molecular and cellular factors in the development and growth of primary brain tumors. To thrive, glioma cells exploit pathways that are active in normal CNS progenitor cells, as well as in normal neurotransmitter signaling. Furthermore, tumor cells of incurable gliomas integrate into communicating multicellular networks, where they are interconnected through neurite-like cellular protrusions. In this Review, we discuss evidence that CNS development, organization and function share a number of common features with glioma progression and malignancy. These include mechanisms used by cells to proliferate and migrate, interact with their microenvironment and integrate into multicellular networks. The emerging intersections between the fields of neuroscience and neuro-oncology considered in this review point to new research directions and novel therapeutic opportunities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
19 November 2019
When this article was initially published online, the Editorial Summary was missing. It should read: “Malignant gliomas recapitulate steps in neurodevelopment to form organ-like structures. Jung et al. review how neuroscience can provide novel insights into glioma biology, and how these insights might be used for future therapeutic approaches.”
References
Arora, R. S. et al. Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro-oncol. 11, 403–413 (2009).
Ostrom, Q. T. et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the united states in 2009-2013. Neuro-oncol. 18(suppl_5), v1–v75 (2016).
Jones, C., Perryman, L. & Hargrave, D. Paediatric and adult malignant glioma: close relatives or distant cousins? Nat. Rev. Clin. Oncol. 9, 400–413 (2012).
Louis, D. N. et al. The2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820 (2016).
Weller, M. et al. Glioma. Nat. Rev. Dis. Primers 1, 15017 (2015).
Filbin, M. & Monje, M. Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat. Med. 25, 367–376 (2019).
Laug, D., Glasgow, S. M. & Deneen, B. A glial blueprint for gliomagenesis. Nat. Rev. Neurosci. 19, 393–403 (2018).
Lim, D. A. & Alvarez-Buylla, A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 8, a018820 (2016).
Sanai, N. et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427, 740–744 (2004).
Sanai, N. et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382–386 (2011).
Paredes, M. F. et al. Extensive migration of young neurons into the infant human frontal lobe. Science 354, aaf7073 (2016).
Bjornsson, C. S., Apostolopoulou, M., Tian, Y. & Temple, S. It takes a village: constructing the neurogenic niche. Dev. Cell 32, 435–446 (2015).
Alvarez-Buylla, A., Kohwi, M., Nguyen, T. M. & Merkle, F. T. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb. Symp. Quant. Biol. 73, 357–365 (2008).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Riquelme, P. A., Drapeau, E. & Doetsch, F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Phil. Trans. R. Soc. Lond. B 363, 123–137 (2008).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 (2003).
Galli, R. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 64, 7011–7021 (2004).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007).
Charles, N. et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6, 141–152 (2010).
Charles, N. & Holland, E. C. The perivascular niche microenvironment in brain tumor progression. Cell Cycle 9, 3012–3021 (2010).
Doetsch, F., García-Verdugo, J. M. & Alvarez-Buylla, A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl Acad. Sci. USA 96, 11619–11624 (1999).
Daynac, M. et al. Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem Cell Res. 11, 516–528 (2013).
Shankar, A. et al. Subcurative radiation significantly increases cell proliferation, invasion, and migration of primary glioblastoma multiforme in vivo. Chin. J. Cancer 33, 148–158 (2014).
Gao, X., McDonald, J. T., Hlatky, L. & Enderling, H. Acute and fractionated irradiation differentially modulate glioma stem cell division kinetics. Cancer Res. 73, 1481–1490 (2013).
Larjavaara, S. et al. Incidence of gliomas by anatomic location. Neuro-oncol. 9, 319–325 (2007).
Barami, K. et al. Relationship of gliomas to the ventricular walls. J. Clin. Neurosci. 16, 195–201 (2009).
Siebzehnrubl, F. A., Reynolds, B. A., Vescovi, A., Steindler, D. A. & Deleyrolle, L. P. The origins of glioma: e pluribus unum? Glia 59, 1135–1147 (2011).
Stiles, C. D. & Rowitch, D. H. Glioma stem cells: a midterm exam. Neuron 58, 832–846 (2008).
Lee, J. H. et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 560, 243–247 (2018).
Platel, J. C., Stamboulian, S., Nguyen, I. & Bordey, A. Neurotransmitter signaling in postnatal neurogenesis: the first leg. Brain Res. Rev. 63, 60–71 (2010).
Nguyen, L. et al. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J. Neurosci. 23, 3278–3294 (2003).
Song, J. et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489, 150–154 (2012).
Liu, X., Wang, Q., Haydar, T. F. & Bordey, A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 8, 1179–1187 (2005).
Ge, S. et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 (2006).
Wang, D. D., Krueger, D. D. & Bordey, A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J. Neurophysiol. 90, 2291–2302 (2003).
Fernando, R. N. et al. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl Acad. Sci. USA 108, 5837–5842 (2011).
Alfonso, J., Le Magueresse, C., Zuccotti, A., Khodosevich, K. & Monyer, H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10, 76–87 (2012).
Dumitru, I., Neitz, A., Alfonso, J. & Monyer, H. Diazepam binding inhibitor promotes stem cell expansion controlling environment-dependent neurogenesis. Neuron 94, 125–137.e5 (2017).
Labrakakis, C., Patt, S., Hartmann, J. & Kettenmann, H. Functional GABA(A) receptors on human glioma cells. Eur. J. Neurosci. 10, 231–238 (1998).
Garzon-Muvdi, T. et al. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol. 10, e1001320 (2012).
Blanchart, A. et al. Endogenous GABAA receptor activity suppresses glioma growth. Oncogene 36, 777–786 (2017).
Alho, H., Kolmer, M., Harjuntausta, T. & Helén, P. Increased expression of diazepam binding inhibitor in human brain tumors. Cell Growth Differ. 6, 309–314 (1995).
Smits, A. et al. GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. PLoS One 7, e37041 (2012).
Duman, C. et al. Acyl-CoA-binding protein drives glioblastoma tumorigenesis by sustaining fatty acid oxidation. Cell Metab. 30, 274–289.e5 (2019).
Jansson, L. C. & Åkerman, K. E. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J. Neural Transm. (Vienna) 121, 819–836 (2014).
Platel, J. C. et al. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 65, 859–872 (2010).
Brazel, C. Y., Nuñez, J. L., Yang, Z. & Levison, S. W. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience 131, 55–65 (2005).
Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8, 189–198 (1992).
Hollmann, M., Hartley, M. & Heinemann, S. Ca2+ permeability of KA-AMPA—gated glutamate receptor channels depends on subunit composition. Science 252, 851–853 (1991).
Darcy, D. P. & Isaacson, J. S. Calcium-permeable AMPA receptors mediate glutamatergic signaling in neural precursor cells of the postnatal olfactory bulb. J. Neurophysiol. 103, 1431–1437 (2010).
Gallo, V. et al. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J. Neurosci. 16, 2659–2670 (1996).
Gudz, T. I., Komuro, H. & Macklin, W. B. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J. Neurosci. 26, 2458–2466 (2006).
Whitney, N. P. et al. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. FASEB J. 22, 2888–2900 (2008).
Wiltgen, B. J. et al. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS One 5, e12818 (2010).
de Groot, J. F., Piao, Y., Lu, L., Fuller, G. N. & Yung, W. K. Knockdown of GluR1 expression by RNA interference inhibits glioma proliferation. J. Neurooncol. 88, 121–133 (2008).
Ishiuchi, S. et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat. Med. 8, 971–978 (2002).
van Vuurden, D. G. et al. Attenuated AMPA receptor expression allows glioblastoma cell survival in glutamate-rich environment. PLoS One 4, e5953 (2009).
Maas, S., Patt, S., Schrey, M. & Rich, A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc. Natl Acad. Sci. USA 98, 14687–14692 (2001).
Rzeski, W., Turski, L. & Ikonomidou, C. Glutamate antagonists limit tumor growth. Proc. Natl Acad. Sci. USA 98, 6372–6377 (2001).
Colman, H. et al. A multigene predictor of outcome in glioblastoma. Neuro-oncol. 12, 49–57 (2010).
Kawahara, Y., Ito, K., Sun, H., Kanazawa, I. & Kwak, S. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur. J. Neurosci. 18, 23–33 (2003).
Arcella, A. et al. Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro-oncol. 7, 236–245 (2005).
Gillespie, S. & Monje, M. An active role for neurons in glioma progression: making sense of Scherer’s structures. Neuro-oncol. 20, 1292–1299 (2018).
Ye, Z. C. & Sontheimer, H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 59, 4383–4391 (1999).
Berg, D. A., Belnoue, L., Song, H. & Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140, 2548–2561 (2013).
Diamandis, P. et al. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat. Chem. Biol. 3, 268–273 (2007).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Barres, B. A. & Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993).
Paul, A., Chaker, Z. & Doetsch, F. Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356, 1383–1386 (2017).
Bergles, D. E., Roberts, J. D., Somogyi, P. & Jahr, C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000).
Lin, S. C. et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46, 773–785 (2005).
Etxeberria, A., Mangin, J. M., Aguirre, A. & Gallo, V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat. Neurosci. 13, 287–289 (2010).
Gautier, H. O. et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6, 8518 (2015).
Zonouzi, M. et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682 (2015).
Fröhlich, N., Nagy, B., Hovhannisyan, A. & Kukley, M. Fate of neuron-glia synapses during proliferation and differentiation of NG2 cells. J. Anat. 219, 18–32 (2011).
Kougioumtzidou, E. et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6, e28080 (2017).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through Neuroligin-3 secretion. Cell 161, 803–816 (2015).
Venkatesh, H. S. et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017).
Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019).
Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019).
de Groot, J. & Sontheimer, H. Glutamate and the biology of gliomas. Glia 59, 1181–1189 (2011).
Parent, J. M., Valentin, V. V. & Lowenstein, D. H. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J. Neurosci. 22, 3174–3188 (2002).
Buckingham, S. C. et al. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 17, 1269–1274 (2011).
Vecht, C. et al. Seizure response to perampanel in drug-resistant epilepsy with gliomas: early observations. J. Neurooncol. 133, 603–607 (2017).
Robel, S., Berninger, B. & Götz, M. The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 12, 88–104 (2011).
Ming, G. L. & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011).
Tsai, H. H. et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351, 379–384 (2016).
Jung, E. et al. Tweety-homolog 1 drives brain colonization of gliomas. J. Neurosci. 37, 6837–6850 (2017).
Osswald, M. et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015).
Lowery, L. A. & Van Vactor, D. The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10, 332–343 (2009).
Walker, T. L., Yasuda, T., Adams, D. J. & Bartlett, P. F. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J. Neurosci. 27, 3734–3742 (2007).
Boulanger, J. J. & Messier, C. Doublecortin in oligodendrocyte precursor cells in the adult mouse brain. Front. Neurosci. 11, 143 (2017).
Daou, M. C., Smith, T. W., Litofsky, N. S., Hsieh, C. C. & Ross, A. H. Doublecortin is preferentially expressed in invasive human brain tumors. Acta Neuropathol. 110, 472–480 (2005).
Koizumi, H. et al. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat. Neurosci. 9, 779–786 (2006).
Suzuki, M. The Drosophila tweety family: molecular candidates for large-conductance Ca2+-activated Cl- channels. Exp. Physiol. 91, 141–147 (2006).
Darmanis, S. et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21, 1399–1410 (2017).
Cao, X. L. et al. Expression and purification of mouse Ttyh1 fragments as antigens to generate Ttyh1-specific monoclonal antibodies. Protein Expr. Purif. 130, 81–89 (2017).
Wiernasz, E. et al. Ttyh1 protein is expressed in glia in vitro and shows elevated expression in activated astrocytes following status epilepticus. Neurochem. Res. 39, 2516–2526 (2014).
Tsai, J. W., Bremner, K. H. & Vallee, R. B. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970–979 (2007).
Habela, C. W., Ernest, N. J., Swindall, A. F. & Sontheimer, H. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J. Neurophysiol. 101, 750–757 (2009).
Sahm, F. et al. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 69, 523–526 (2012).
Le Magueresse, C. et al. Subventricular zone-derived neuroblasts use vasculature as a scaffold to migrate radially to the cortex in neonatal mice. Cereb. Cortex 22, 2285–2296 (2012).
Winkler, F. et al. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 57, 1306–1315 (2009).
Griveau, A. et al. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 33, 874–889.e7 (2018).
Farin, A. et al. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53, 799–808 (2006).
Qin, E. Y. et al. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell 170, 845–859.e19 (2017).
Brose, K. et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806 (1999).
Guerrero-Cazares, H. et al. Brief report: Robo1 regulates the migration of human subventricular zone neural progenitor cells during development. Stem Cells 35, 1860–1865 (2017).
Yiin, J. J. et al. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro-oncol. 11, 779–789 (2009).
Giese, A., Bjerkvig, R., Berens, M. E. & Westphal, M. Cost of migration: invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 21, 1624–1636 (2003).
Campbell, S. L. et al. GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia 63, 23–36 (2015).
Winkler, F. & Wick, W. Harmful networks in the brain and beyond. Science 359, 1100–1101 (2018).
Osswald, M., Solecki, G., Wick, W. & Winkler, F. A malignant cellular network in gliomas: potential clinical implications. Neuro-oncol. 18, 479–485 (2016).
Devoto, S. H. Neuronal growth cone migration. Experientia 46, 916–922 (1990).
Marín, O., Valiente, M., Ge, X. & Tsai, L. H. Guiding neuronal cell migrations. Cold Spring Harb. Perspect. Biol. 2, a001834 (2010).
Skene, J. H. et al. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science 233, 783–786 (1986).
Aigner, L. & Caroni, P. Absence of persistent spreading, branching, and adhesion in GAP-43-depleted growth cones. J. Cell Biol. 128, 647–660 (1995).
Haag, D. et al. Nos2 inactivation promotes the development of medulloblastoma in Ptch1(+/-) mice by deregulation of Gap43-dependent granule cell precursor migration. PLoS Genet. 8, e1002572 (2012).
Aigner, L. et al. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83, 269–278 (1995).
Zuber, M. X., Goodman, D. W., Karns, L. R. & Fishman, M. C. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science 244, 1193–1195 (1989).
Stefaniuk, M., Swiech, L., Dzwonek, J. & Lukasiuk, K. Expression of Ttyh1, a member of the Tweety family in neurons in vitro and in vivo and its potential role in brain pathology. J. Neurochem. 115, 1183–1194 (2010).
Mohiuddin, L., Fernandez, K., Tomlinson, D. R. & Fernyhough, P. Nerve growth factor and neurotrophin-3 enhance neurite outgrowth and up-regulate the levels of messenger RNA for growth-associated protein GAP-43 and T alpha 1 alpha-tubulin in cultured adult rat sensory neurones. Neurosci. Lett. 185, 20–23 (1995).
Benowitz, L. I. & Routtenberg, A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20, 84–91 (1997).
Peinado, A. Immature neocortical neurons exist as extensive syncitial networks linked by dendrodendritic electrical connections. J. Neurophysiol. 85, 620–629 (2001).
Scemes, E. & Giaume, C. Astrocyte calcium waves: what they are and what they do. Glia 54, 716–725 (2006).
Leybaert, L. & Sanderson, M. J. Intercellular Ca(2+) waves: mechanisms and function. Physiol. Rev. 92, 1359–1392 (2012).
Kuga, N., Sasaki, T., Takahara, Y., Matsuki, N. & Ikegaya, Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 31, 2607–2614 (2011).
Kraft, A. et al. Astrocytic calcium waves signal brain injury to neural stem and progenitor cells. Stem Cell Rep. 8, 701–714 (2017).
Doetsch, F., Caillé, I., Lim, D. A., García-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Lacar, B., Young, S. Z., Platel, J. C. & Bordey, A. Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur. J. Neurosci. 34, 1895–1905 (2011).
Ravella, A., Ringstedt, T., Brion, J. P., Pandolfo, M. & Herlenius, E. Adult neural precursor cells form connexin-dependent networks that improve their survival. Neuroreport 26, 928–936 (2015).
Malmersjö, S. et al. Neural progenitors organize in small-world networks to promote cell proliferation. Proc. Natl Acad. Sci. USA 110, E1524–E1532 (2013).
Malmersjö, S., Rebellato, P., Smedler, E. & Uhlén, P. Small-world networks of spontaneous Ca(2+) activity. Commun. Integr. Biol. 6, e24788 (2013).
Murphy, S. F. et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 76, 139–149 (2016).
Wang, J. et al. Targeting different domains of gap junction protein to control malignant glioma. Neuro-oncol. 20, 885–896 (2018).
Weil, S. et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro-oncol. 19, 1316–1326 (2017).
Le, H. T. et al. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J. Biol. Chem. 289, 1345–1354 (2014).
Rustom, A. The missing link: does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol. 6, 160057 (2016).
Ariazi, J. et al. Tunneling nanotubes and gap junctions-their role in long-range intercellular communication during development, health, and disease conditions. Front. Mol. Neurosci. 10, 333 (2017).
Wesseling, P., van den Bent, M. & Perry, A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol. 129, 809–827 (2015).
Barabási, A. L. & Oltvai, Z. N. Network biology: understanding the cell’s functional organization. Nat. Rev. Genet. 5, 101–113 (2004).
Wang, X., Mao, X., Xie, L., Greenberg, D. A. & Jin, K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 29, 1644–1654 (2009).
Goings, G. E., Sahni, V. & Szele, F. G. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 996, 213–226 (2004).
Kojima, T. et al. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28, 545–554 (2010).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 (2002).
Kreuzberg, M. et al. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp. Neurol. 226, 90–99 (2010).
Inta, D. & Gass, P. Is forebrain neurogenesis a potential repair mechanism after stroke? J. Cereb. Blood Flow Metab. 35, 1220–1221 (2015).
Huttner, H. B. et al. The age and genomic integrity of neurons after cortical stroke in humans. Nat. Neurosci. 17, 801–803 (2014).
Alieva, M. et al. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci. Rep. 7, 7529 (2017).
Chen, W. et al. Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med. Oncol. 32, 43 (2015).
Author information
Authors and Affiliations
Contributions
E.J., J.A., M.O., W.W., H.M., and F.W. wrote the manuscript. E.J. created the figures. J.A. acquired the immunofluorescence image of the SVZ. E.J. acquired the two-photon microscopy image of the glioma cells in vivo.
Corresponding author
Ethics declarations
Competing interests
E.J., F.W., and W.W. report the patent (WO2017020982A1) “Agents for use in the treatment of glioma.” F.W. is co-founder of DC Europa Ltd (a company trading under the name Divide & Conquer), which is developing new medicines for the treatment of glioma. Divide & Conquer also provides research funding to F.W.’s lab under a research collaboration agreement.
Additional information
Peer review information Nature Neuroscience thanks Benjamin Deneen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, E., Alfonso, J., Osswald, M. et al. Emerging intersections between neuroscience and glioma biology. Nat Neurosci 22, 1951–1960 (2019). https://doi.org/10.1038/s41593-019-0540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0540-y
This article is cited by
-
A clinically applicable connectivity signature for glioblastoma includes the tumor network driver CHI3L1
Nature Communications (2024)
-
Impact of GAP-43, Cx43 and actin expression on the outcome and overall survival in diffuse and anaplastic gliomas
Scientific Reports (2023)
-
Duchenne muscular dystrophy gene expression is an independent prognostic marker for IDH mutant low-grade glioma
Scientific Reports (2022)
-
CDC42EP3 promotes glioma progression via regulation of CCND1
Cell Death & Disease (2022)
-
Insights and opportunities at the crossroads of cancer and neuroscience
Nature Cell Biology (2022)