Abstract
Key message
Benzoate-Coenzyme A ligase enzyme activity catalyzing the conversion of free benzoic acid to benzoyl-CoA was detected and biochemically characterized in the elicitor-treated pear cell cultures.
Abstract
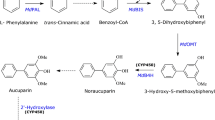
Asian pear (Pyrus pyrifolia) is an economically and nutritionally important fruit-bearing tree of the subtribe Malinae. Upon pathogen attack, pears produce unique benzoate-derived biphenyl phytoalexins. The upstream biosynthesis of the biphenyl in Malinae is still incomplete. Previously, protein preparations from yeast extract-treated pear cultures were able to convert l-phenylalanine to cinnamic acid catalyzed by the activity of the phenylalanine ammonia lyase. The same extract was able to perform a C2 side-chain cleavage of cinnamic acid to benzaldehyde followed by oxidation of the latter to benzoic acid owing to the molecularly-undefined benzaldehyde synthase and benzaldehyde dehydrogenase activities, respectively. The biosynthesis of biphenyls starts with benzoate-Coenzyme A ligase (BZL), which converts benzoic acid to benzoyl-CoA. Subsequently, the previously-defined biphenyl synthase uses benzoyl-CoA to form the biphenyls. The current study reports the first time detection and characterization of BZL activity in elicitor-treated pear cell cultures. The preferred substrate was benzoic acid (Km = 62 ± 4 µM). Magnesium or manganese was prerequisite for the activity, which was enhanced by ~ 70% in the presence of potassium. Maximum BZL activity was observed 18 h post elicitation, which is in agreement with the coordinate induction reported for the enzymes in the same pathway. The induced BZL activity preceded the accumulation of biphenyls supporting its involvement in their biosynthesis.




Similar content being viewed by others
References
Abd El-Mawla AM, Beerhues L (2002) Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta 214:727–733. https://doi.org/10.1007/s004250100657
Barillas W, Beerhues L (1997) 3-Hydroxybenzoate:coenzyme A ligase and 4-coumarate: coenzyme A ligase from cultured cells of Centaurium erythraea. Planta 202:112–116
Barillas W, Beerhues L (2000) 3-Hydroxybenzoate:coenzyme A ligase from cell cultures of Centaurium erythraea: isolation and characterization. Biol Chem 381:155–160
Beuerle T, Pichersky E (2002a) Enzymatic synthesis and purification of aromatic coenzyme A esters. Anal Biochem 302:305–312
Beuerle T, Pichersky E (2002b) Purification and characterization of benzoate:coenzyme A ligase from Clarkia breweri. Arch Biochem Biophys 400:258–264
Bjorklund JA, Leete E (1992) Biosynthesis of the benzoyl moiety of cocaine from cinnamic acid via (R)-(+)-3-hydroxy-3-phenylpropanoic acid. Phytochemistry 31:3883–3887. https://doi.org/10.1016/S0031-9422(00)97546-0
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bussell JD, Reichelt M, Wiszniewski AAG et al (2014) Peroxisomal ATP-binding cassette transporter comatose and the multifunctional protein abnormal inflorescence meristem are required for the production of benzoylated metabolites in Arabidopsis seeds. Plant Physiol 164:48–54. https://doi.org/10.1104/pp.113.229807
Chizzali C, Beerhues L (2012) Phytoalexins of the pyrinae: biphenyls and dibenzofurans. Beilstein J Org Chem 8:613–620. https://doi.org/10.3762/bjoc.8.68
Chizzali C, Khalil MNA, Beuerle T et al (2012) Formation of biphenyl and dibenzofuran phytoalexins in the transition zones of fire blight-infected stems of Malus domestica cv. “Holsteiner Cox” and Pyrus communis cv. “Conference”. Phytochemistry 77:179–185. https://doi.org/10.1016/j.phytochem.2012.01.023
Chizzali C, Swiddan AK, Abdelaziz S et al (2016) Expression of biphenyl synthase genes and formation of phytoalexin compounds in three fire blight-infected Pyrus communis cultivars. PLoS One 11:e0158713. https://doi.org/10.1371/journal.pone.0158713
Colquhoun TA, Marciniak DM, Wedde AE et al (2012) A peroxisomally localized acyl-activating enzyme is required for volatile benzenoid formation in a Petunia × hybrida cv. “Mitchell Diploid” flower. J Exp Bot 63:4821–4833. https://doi.org/10.1093/jxb/ers153
Fotirić Akšić MM, Dabić DČ, Gašić UM et al (2015) Polyphenolic profile of pear leaves with different resistance to Pear Psylla (Cacopsylla pyri). J Agric Food Chem 63:7476–7486. https://doi.org/10.1021/acs.jafc.5b03394
Gaid MM, Scharnhop H, Ramadan H et al (2011) 4-Coumarate:coA ligase family members from elicitor-treated Sorbus aucuparia cell cultures. J Plant Physiol 168:944–951. https://doi.org/10.1016/j.jplph.2010.11.021
Gaid MM, Sircar D, Muller A et al (2012) Cinnamate:coA ligase initiates the biosynthesis of a benzoate-derived xanthone phytoalexin in Hypericum calycinum Cell Cultures. Plant Physiol 160:1267–1280. https://doi.org/10.1104/pp.112.204180
Hüttner C, Beuerle T, Scharnhop H, Ernst L, Beerhues L (2010) Differential effect of elicitors on biphenyl and dibenzofuran formation in sorbus aucuparia cell cultures. J Agric Food Chem 58:11977-11984.
Jiang S, Zheng X, Yu P et al (2016) Primitive genepools of asian pears and their complex hybrid origins inferred from fluorescent sequence-specific amplification polymorphism (SSAP) markers based on LTR retrotransposons. PLoS One 11:e0149192. https://doi.org/10.1371/journal.pone.0149192
Khalil MNA, Brandt W, Beuerle T et al (2015) O-Methyltransferases involved in biphenyl and dibenzofuran biosynthesis. Plant J 83:263–276. https://doi.org/10.1111/tpj.12885
Klempien A, Kaminaga Y, Qualley A et al (2012) Contribution of CoA ligases to benzenoid biosynthesis in Petunia flowers. Plant Cell 24:2015–2030. https://doi.org/10.1105/tpc.112.097519
Liu B, Beuerle T, Klundt T, Beerhues L (2004) Biphenyl synthase from yeast-extract-treated cell cultures of Sorbus aucuparia. Planta 218:492–496. https://doi.org/10.1007/s00425-003-1144-y
Qualley AV, Widhalm JR, Adebesin F et al (2012) Completion of the core -oxidative pathway of benzoic acid biosynthesis in plants. Proc Natl Acad Sci 109:16383–16388. https://doi.org/10.1073/pnas.1211001109
Saini SS, Teotia D, Gaid M et al (2017) Benzaldehyde dehydrogenase-driven phytoalexin biosynthesis in elicitor-treated Pyrus pyrifolia cell cultures. J Plant Physiol 215:154–162. https://doi.org/10.1016/j.jplph.2017.06.004
Saini SS, Teotia D, Gaid M, Sircar D (2019) A new enzymatic activity from elicitor-treated pear cell cultures converting trans-cinnamic acid to benzaldehyde. Physiol Plant 167:64–74. https://doi.org/10.1111/ppl.12871
Sarkate A, Banerjee S, Mir JI, Roy P, Sircar D (2017) Antioxidant and cytotoxic activity of bioactive phenolic metabolites isolated from the yeast-extract treated cell culture of apple. Plant Cell, Tissue Organ Cult 130:641–664
Sarkate A, Saini SS, Teotia D, Gaid M, Mir JI, Roy P, Agrawal PK, Sircar D (2018) Comparative metabolomics of scab-resistance and susceptible apple cell cultures in response to scab fungus elicitor treatment. Sci Reoprts 8:17844
Sarkate A, Saini SS, Gaid M, Teotia D, Mir JI, Agrawal PK, Beerhues L, Sircar D (2019) Molecular cloning and functional analysis of a biphenyl phytoalexin-specific O-methyltransferase from apple cell suspension cultures. Planta 249:677. https://doi.org/10.1007/s00425-018-3031-6
Schmelz S, Naismith JH (2009) Adenylate-forming enzymes. Curr Opin Struct Biol 19:666–671
Sircar D, Gaid M, Chizzali C et al (2015) Biphenyl 4-hydroxylases involved in aucuparin biosynthesis in Rowan and Apple are CYP736A proteins. Plant Physiol 168:428–442. https://doi.org/10.1104/pp.15.00074
Stewart CJ, Woods K, Macias G, Allan AC, Hellens RP, Noel JP (2017) Molecular architectures of benzoic acid-specific type III polyketide synthases. Acta Crystallogr D Struct Biol 73:1007–1019. https://doi.org/10.1107/S2059798317016618
Stuible HP, Büttner D, Ehlting J et al (2000) Mutational analysis of 4-coumarate:coA ligase identifies functionally important amino acids and verifies its close relationship to other adenylate-forming enzymes. FEBS Lett 467:117–122. https://doi.org/10.1016/S0014-5793(00)01133-9
Teng Y, Tanabe K, Tamura F, Itai A (2002) Genetic relationships of Pyrus species and cultivars native to east asia revealed by randomly amplified polymorphic DNA markers. J Am Soc Hortic Sci 127:262–270. https://doi.org/10.21273/jashs.127.2.262
Teotia D, Gaid M, Saini SS et al (2019) Cinnamate-CoA ligase is involved in biosynthesis of benzoate-derived biphenyl phytoalexin in Malus domestica ‘Golden Delicious’ cell cultures. Plant J. https://doi.org/10.1111/tpj.14506
Van Moerkercke A, Schauvinhold I, Pichersky E et al (2009) A plant thiolase involved in benzoic acid biosynthesis and volatile benzenoid production. Plant J 60:292–302. https://doi.org/10.1111/j.1365-313X.2009.03953.x
Acknowledgements
This research work was supported by a start up research grant (FIG 100624 to D. Sircar) from the Indian Institute of Technology Roorkee. SSS is thankful to MHRD-research assistantship (MHRD02-23-200-429) from Indian Institute of Technology Roorkee for perusing his doctoral studies. We thank Dr. Till Beuerle (Technische Universität Braunschweig, Germany) for ESI–MS analyses.
Author information
Authors and Affiliations
Contributions
DS conceived and designed the study. SSS performed experiments, data processing and analyses. MG and DS performed data analyses and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Baochun Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saini, S.S., Gaid, M. & Sircar, D. Benzoate-CoA ligase contributes to the biosynthesis of biphenyl phytoalexins in elicitor-treated pear cell cultures. Plant Cell Rep 39, 207–215 (2020). https://doi.org/10.1007/s00299-019-02484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02484-0