Abstract
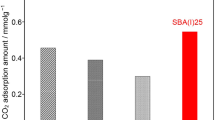
In this work, silica-based CO2 adsorbents were successfully prepared by the sol–gel method. These materials were chemically modified with 3-aminopropyltriethoxysilane (APTES) using the grafting technique. Two synthesis parameters were investigated in this study, namely the solvent used in the grafting step (toluene against ethanol) and the process employed for removing the solvent (filtration against evaporation). The influence of these parameters on pore structure, surface chemistry and CO2 capture performance were evaluated and discussed on the basis of a series of experimental tests, including FTIR, TG-MS, nitrogen adsorption tests, XRD, TEM and CO2 adsorption. The use of ethanol led to samples with a NH2 concentration of about 1.20 ± 0.05 mmol g−1. On the other hand, samples obtained using toluene showed amine concentrations two times higher than the specimens prepared with ethanol. Samples obtained by evaporation exhibited blocked pores and a low CO2 adsorption capacity when compared to those obtained by filtration. The sample prepared using toluene as the solvent and a filtration step displayed a CO2 adsorption capacity as high as 6.3 wt% at 30 °C and 1 bar.














Similar content being viewed by others
References
Massachussets Institute of Technology, The Future of Natural Gas: An Interdisciplinary MIT Study (Massachussets Institute of Technology, Cambridge, 2011)
X. Liu, L. Zhou, X. Fu, Y. Sun, W. Su, Y. Zhou, Chem. Eng. Sci. 62, 1101 (2007)
R. Kishor, A.K. Ghoshal, Chem. Eng. J. 262, 882 (2015)
Y. Belmabkhout, R. Serna-guerrero, A. Sayari, Adsorpt. J. Int. Adsorpt. Soc. 49, 359 (2010)
Y. Belmabkhout, G. De Weireld, A. Sayari, Langmuir 25, 13275 (2009)
Y.G. Ko, S.S. Shin, U.S. Choi, J. Colloid Interface Sci. 361, 594 (2011)
J. Wei, J. Shi, H. Pan, W. Zhao, Q. Ye, Y. Shi, Microporous Mesoporous Mater. 116, 394 (2008)
L. Mafra, T. Čendak, S. Schneider, P.V. Wiper, J. Pires, J.R.B. Gomes, M.L. Pinto, Chem. Eng. J. 336, 612 (2018)
C.-H. Yu, C.-H. Huang, C.-S. Tan, Aerosol Air. Qual. Res. 12, 745 (2012)
E.F. Vansant, P.V. Voort, K.C. Vrancken (eds.), Characterization and chemical modification of the silica surface (Elsevier, Amsterdam, 1995), pp. 193–297
A.C.C. Chang, S.S.C. Chuang, M. Gray, Y. Soong, Energy Fuels 17, 468–473 (2003)
A. Simon, T. Cohen-Bouhacina, M.C. Port, J.P. Aim, J. Colloid Interface Sci. 283, 278 (2002)
P. Shah, N. Sridevi, A. Prabhune, V. Ramaswamy, Microporous Mesoporous Mater. 116, 157 (2008)
M. Chaimberg, Y. Cohen, J. Colloid Interface Sci. 134, 576 (1990)
F. Cuoq, A. Masion, J. Labille, J. Rose, F. Ziarelli, B. Prelot, J. Bottero, Appl. Surf. Sci. 266, 155 (2013)
W.J.D. Ng, Z. Zhong, J. Luo, A. Borgna, Int. J. Hydrogen Energy 35, 12724 (2010)
H. He, J. Duchet, J. Galy, J. Gerard, J. Colloid Interface Sci. 288, 171 (2005)
A. Krysztafkiewicz, T. Jesionowski, S. Binkowski, Colloids Surfaces A Physicochem. Eng. Asp. 173, 73 (2000)
Z. Bahrami, A. Badiei, F. Atyabi, Chem. Eng. Res. Des. 2, 1296 (2013)
M. Moritz, M. Łaniecki, Appl. Surf. Sci. 258, 7523 (2012)
V. Hernández-Morales, R. Nava, Y.J. Acosta-Silva, S.A. MacÍas-Sánchez, J.J. Pérez-Bueno, B. Pawelec, Microporous Mesoporous Mater. 160, 133 (2012)
A.Z. Abdullah, N.S. Sulaiman, A.H. Kamaruddin, Biochem. Eng. J. 44, 263 (2009)
P.T.B. Nguyen, J. Lee, W.G. Shim, H. Moon, Microporous Mesoporous Mater. 110, 560 (2008)
S. Shylesh, A.P. Singh, J. Catal. 244, 52 (2006)
Q. Xue, Y. Liu, J. Ind. Eng. Chem. 18, 169 (2012)
X. Zhang, H. Qin, X. Zheng, W. Wu, Mater. Res. Bull. 48, 3981 (2013)
B.M. Yue, B.L. Sun, Z.J. Wang, Y. Wang, Q. Yu, J.H. Zhu, Microporous Mesoporous Mater. 114, 74 (2008)
A. Zhao, A. Samanta, P. Sarkar, R. Gupta, Ind. Eng. Chem. Res. 52, 6480 (2013)
J. Kim, R.J. Desch, S.W. Thiel, V.V. Guliants, N.G. Pinto, J. Chromatogr. A 1218, 7796 (2011)
V. Meynen, P. Cool, E.F. Vansant, Microporous Mesoporous Mater. 125, 170 (2009)
H.M. Alsyouri, M.A. Abu-Daabes, A. Alassali, J.Y. Lin, Nanoscale Res. Lett. 8, 484 (2013)
P.F. Fulvio, S. Pikus, M. Jaroniec, J. Mater. Chem. 15, 5049 (2005)
Y. Liu, T.J. Pinnavaia, J. Mater. Chem. 12, 3179 (2002)
A. Galarneau, H. Cambon, F. Di Renzo, R. Ryoo, M. Choi, F. Fajula, New J. Chem. 27, 73 (2002)
K. Cassiers, T. Linssen, M. Mathieu, M. Benjelloun, K. Schrijnemakers, P. Van Der Voort, P. Cool, E.F. Vansant, Chem. Mater. 14, 2317 (2002)
C.P. Tripp, M.L. Hair, Langmuir 7, 923 (1991)
J. Román, S. Padilla, M. Vallet-Regí, Chem. Mater. 15, 798 (2003)
B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, New York, 1998)
A. del Campo, T. Sen, J.-P. Lellouche, I.J. Bruce, J. Magn. Magn. Mater. 293, 33 (2005)
S. Xie, M. Gan, L. Ma, Z. Li, J. Yan, H. Yin, X. Shen, F. Xu, J. Zheng, J. Zhang, J. Hu, Electrochim. Acta 120, 408 (2014)
R.M. Almeida, C.G. Pantano, J. Appl. Phys. 68, 4225 (1990)
V. Zelenak, D. Halamova, L. Gaberova, E. Bloch, P.L. Llewellyn, Microporous Mesoporous Mater. 116, 358 (2008)
L. Wang, L. Ma, A. Wang, Q. Liu, T. Zhang, Chinese. J. Catal. 28, 805 (2007)
F.Y. Chang, K.J. Chao, H.H. Cheng, C.S. Tan, Sep. Purif. Technol. 70, 87 (2009)
E. Da’na, A. Sayari, Chem. Eng. J. 166, 445 (2011)
J.M. Rosenholm, M. Lindén, Chem. Mater. 19, 5023 (2007)
V. Zelenák, M. Badanicová, D. Halamová, J. Cejka, A. Zukal, N. Murafa, G. Goerigk, Chem. Eng. J. 144, 336 (2008)
S. Saravanamurugan, D. Han, J. Koo, S. Park, Catal. Commun. 9, 158 (2008)
Y. Li, N. Sun, L. Li, N. Zhao, F. Xiao, W. Wei, Y. Sun, W. Huang, Materials (Basel). 6, 981 (2013)
S.A. Didas, M.A. Sakwa-novak, G.S. Foo, C. Sievers, C.W. Jones, J. Phys. Chem. Lett. Scheme 5, 4194–4200 (2014)
M. Thommes, K. Kaneko, A.V. Neimark, J.P. Olivier, F. Rodriguez-reinoso, J. Rouquerol, K.S.W. Sing, Pure Appl. Chem. 87, 1051 (2015)
J. Liu, R. Lin, Powder Technol. 241, 188 (2013)
Acknowledgements
The authors thank the financial support from Petrobras, Equinor, and ANP (National Agency of Petroleum, Natural Gas and Biofuel). We also thank the technical support of UFMG Microscopy Center in the TEM tests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de O. N. Ribeiro, J., Nunes, E.H.M., Vasconcelos, D.C.L. et al. Role of the type of grafting solvent and its removal process on APTES functionalization onto SBA-15 silica for CO2 adsorption. J Porous Mater 26, 1581–1591 (2019). https://doi.org/10.1007/s10934-019-00754-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-019-00754-6