Abstract
In humans, early-life adversity is associated with impairments in learning and memory that may emerge later in life. In rodent models, early-life adversity directly impacts hippocampal neuron structure and connectivity with progressive deficits in long-term potentiation and spatial memory function. Previous work has demonstrated that augmented release and actions of the stress-activated neuropeptide, CRH, contribute to the deleterious effects of early-life adversity on hippocampal dendritic arborization, synapse number and memory-function. Early-life adversity increases hippocampal CRH expression, and blocking hippocampal CRH receptor type-1 (CRHR1) immediately following early-life adversity prevented the consequent memory and LTP defects. Here, we tested if blocking CRHR1 in young adults ameliorates early-life adversity-provoked memory deficits later in life. A weeklong course of a CRHR1 antagonist in 2-month-old male rats prevented early-life adversity-induced deficits in object recognition memory that emerged by 12 months of age. Surprisingly, whereas the intervention did not mitigate early-life adversity-induced spatial memory losses at 4 and 8 months, it restored hippocampus-dependent location memory in 12-month-old rats that experienced early-life adversity. Neither early-life adversity nor CRHR1 blockade in the adult influenced anxiety- or depression-related behaviors. Altogether, these findings suggest that cognitive deficits attributable to adversity during early-life-sensitive periods are at least partially amenable to interventions later in life.
Similar content being viewed by others
Introduction
Age-related memory loss has a significant impact on an individual’s quality of life in addition to global economic burden [1, 2]. Predisposition to cognitive disorders throughout life is established through an interplay of inherited and environmental factors [3, 4]. Brain development during the early postnatal period is particularly susceptible to environmental influences [5,6,7,8,9,10] and in rodents the first 2 weeks of life represent a sensitive period for hippocampal maturation [11]. In humans, studies have found association with an impoverished environment during childhood and impaired cognition/dementia later in life [12, 13], however, it is difficult to account for genetic and societal factors in these analyses [14]. Mechanistic studies in rodents have found that stress early in life can lead to delayed, progressive impairments of hippocampal function [15, 16]. These enduring memory deficits are likely due to a cascade of cellular and molecular mechanisms that ultimately result in changes to learning and memory circuits [17,18,19,20].
The hippocampus is particularly vulnerable to adverse experiences early in life. There is evidence of reduced hippocampal volume in children raised in orphanages [21] and other types of adversity [22, 23], and in rodents exposed to early-life stress [15, 24, 25]. In rodents, the reduction in volume is likely a result of reduced dendritic arborization [25,26,27,28]. Reduction in hippocampal dendrites is a well described consequence of chronic stress, glucocorticoids acting via glucocorticoid receptors (GR) [29, 30] and corticotropin releasing hormone (CRH) [16, 28, 31] both impact dendritic arborization. This is thought to involve a process commencing with loss of synapses and dendritic spines and subsequent dendritic atrophy [32, 33]. During development, both glucocorticoids and CRH may directly inhibit dendritic arborization [28, 34]. CRH is expressed in the hippocampus within a subpopulation of interneurons [35,36,37,38,39,40]. Both tonically [41] and during stress, CRH is released locally and binds to corticotropin releasing hormone type-1 (CRHR1) receptors on pyramidal cells [42], resulting in neuronal activation [41,42,43]. Sustained increases in CRHR1 activation in the hippocampus results in destruction of dendritic spines and synapse integrity via actin remodeling [31, 44,45,46], promoting deficits in learning and memory [47,48,49].
A rodent model of simulated poverty accomplished by limiting bedding and nesting materials in pups cages during postnatal days 2–9 (LBN) leads to sustained elevations in CRH in the hippocampus [16, 24]. As adults, rodents who experienced the LBN paradigm (LBN rats or mice) have significant impairments in learning and memory [15, 17, 25, 50,51,52], and these worsen with age [15]. These impairments in learning and memory were replicated by infusing CRH directly into the brains of immature rats while controlling the levels of circulating glucocorticoids [53]. Conversely, when a CRHR1 antagonist is administered during the sensitive period of hippocampal development, deficits in learning and memory following LBN are prevented [24]. This suggests a vital contribution of CRH to the progressive deficits in learning and memory resulting from early-life adversity, and demonstrates that early mechanism-based interventions, immediately following the adversity period, are effective.
The goal of the present study was to identify if interventions later in life, and specifically during young adulthood, can alleviate adversity-induced memory loss and its progression.
Methods
Animals
Subjects were male rats born to timed-pregnant Sprague-Dawley rat dams maintained on 12 h light/ dark cycles with ad libitum access to chow and water. On P2, litters were cross-fostered for all groups to obviate potential genetic and litter size confounders. For experiment 1 (Fig. 1b, c), male and female pups from a total of seven litters were distributed across six dams (three control and three LBN dams) to a maximum of 12 pups per dam. After weaning, males were then randomly assigned to an experimental group (control; untreated (4), vehicle (3), antagonist (5), or LBN; untreated (4), vehicle (3) or antagonist (6)). For experiment 2 (Figs. 2 and 3), male and female pups from four litters were gathered, and assigned at random to three dams (one control and two LBN dams), to a maximum of 12 pups per dam. After weaning, males were randomly assigned to experimental group (control; vehicle (3), antagonist (3) and LBN; vehicle (6), antagonist (6)). Weaned males were housed with littermates of the same treatment group, three per cage. Female animals will be the focus of future studies [54]. The results from experiment 1 (Fig. 1) were used to inform the study design of experiment 2 (Figs. 2 and 3) and the numbers were determined accordingly. All experiments were performed in accordance with National Institutes of Health guidelines and were approved by the University of California-Irvine, Animal Care and Use Committee.
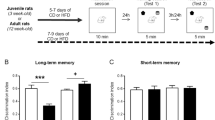
The intervention surgery itself does not influence memory throughout adulthood. a Experimental design with timeline representing the timing of interventions and testing, and the ages at which they occurred. b, c Minipump surgery had no effect on object recognition memory ratio (b) or discrimination index (c) at 10 months of age. n = 3–7 per group, mean with ± SEM, dots represent individual animals
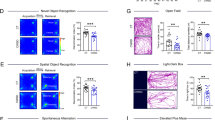
CRHR1 blocker administration ameliorates adversity-provoked memory impairments in a task and age-dependent manner. a Animals were trained on ORM for 10 min, 24 h later one object was changed, and animals were testing for object discrimination for 5 min. b There were no effects of CRHR1 antagonist in any of the groups at 4 or 8 months of age, at 12 months of age there was a significant improvement of object discrimination in LBN animals, which received administration of CRHR1 antagonist. c Plot representing individual values for object preference on ORM. d Animals were trained on new objects for the OLM task, and on testing day, one of these objects was moved to the opposite side of the arena. e There was an overall effect of LBN across the ages on the discrimination of the new location, at 12 months of age there was a significant improvement in DI in the LBN animals, which received antagonist. f Plot representing individual values for object preference on OLM. Control veh n = 3, control antag n = 3, LBN veh n = 6, LBN antagonist n = 6. *p < 0.05 (post-test), for b, e symbols represent mean with ± SEM connected by interpolated lines. For c, f bars represent mean and symbols represented individual values. ORM = object recognition memory, OLM = object location memory, veh = vehicle, antag = CRHR1 antagonist, LBN = limited bedding nesting
Early-life adversity and CRHR1 blocker administration to adult rats have no effects on anxiety- and depression-related phenotypes. There was no effect of LBN or CRHR1 antagonist at any of the ages tested on either % time in the open arm (a) on the elevated plus maze. b Plot representing individual values for arm preference on EPM. c Total immobility time in the forced swim test was not affected by LBN nor CRHR1 antagonist. d Plot representing individual immobility times. Control veh n = 3, control antag n = 3, LBN veh n = 6, LBN antagonist n = 6. *p < 0.05 (post-test for a and c symbols represent mean with ± SEM connected by interpolated lines. For b and d bars represent mean and symbols represented individual values. veh = vehicle, antag = CRHR1 antagonist, LBN = limited bedding nesting
The early-life adversity paradigm
The simulated poverty limited bedding and nesting paradigm (LBN) consisted of limiting nesting and bedding materials in cages between P2–P9 as described previously [55, 56]. Control and experimental cages were undisturbed during P2–P9. One animal in the LBN + CRHR1 antagonist group died prior to testing on the elevated plus maze at 12 months of age and therefore is not included in these analyses (see supplementary methods).
Intracerebroventricular (ICV) administration of CRHR1 blocker
The selective CRHR1 blocker, NBI30775 (3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N,N-dipropyl-pyrazolo[2,3-a]pyrimidin-7-amine) (0.5 µL/hour, ~4 mg/kg/day) or vehicle were chronically infused into the cerebral ventricles via osmotic minipumps (model 2001, Alzet Corp., Cupertino, CA), to both control and LBN male rats for 1 week, commencing at 2 months of age [24] (see Supplementary methods).
Memory tests: novel object recognition memory (ORM) and object location memory (OLM) tests
Tests of object recognition and of spatial memory were conducted at 4, 8 and 12 months of age (Fig. 1a) as described previously [25]. Training consisted of rats exploring two identical objects for 10 min. For recognition memory (ORM) (Fig. 2a), rats were tested 24 h later: they were presented with a duplicate of a previously encountered object from the training session and a novel object. For OLM testing (Fig. 2d), one of the two objects was moved to the center of the cage, and the other object remained in the previous location. Testing sessions lasted for 5 min. The ratio of time spent with novel object or one located in a novel place over the familiar object/location was calculated. In addition, the discrimination index (DI) was calculated as ((novel – familiar)/(novel + familiar)) × 100 as an index of memory in both ORM and OLM tests (see Supplementary methods).
Elevated plus maze (EPM)
To examine the potential influence of early-life adversity on “‘anxiety-like” behaviors, rats were tested on the elevated plus maze for a 5-min trial as described previously [15] and percentage time spent in the open arm was calculated (see Supplementary methods).
Porsolt’s forced swim test (FST)
The swim test consisted of two sessions separated by 24 h in a dimly lit room as described previously [57]. The durations of floating (immobility), climbing, and swimming were scored and served as indicators of depressive-like vs. coping-like behaviors [58] (see Supplementary methods).
Statistical considerations and analyses
The longitudinal assessment of memory (ORM and OLM) over the lifespan of an individual rat was conducted using three-way repeated measures analysis of variances with a Greenhouse-Geisser correction. To account for the missing animal in the 12-month time point, a mixed-effects model (REML) was used to analyze EPM and FST data; significance was set at p ≤ 0.05. T-tests with Benjamini–Hochberg correction wer used for post-hoc tests to determine specific effects of antagonist. Statistical analyses were performed using GraphPad prism 8.0 (GraphPad software, Inc., LA Jolla, CA). All graphs show the mean ± standard error of the mean (SEM).
Results
A CRHR1 blocker administered during early adulthood rescues memory deficits provoked by early-life adversity
The intervention surgery itself does not influence memory throughout adulthood
We first examined for potential effects of the minipump implantation surgery on memory. A separate cohort of animals were reared in LBN cages (n = 16) or in control lab cages (n = 12). As adults, half of each group received osmotic minipumps. Of the surgery animals, six rats received vehicle (three control, three LBN) and 13 received CRHR1 antagonist (seven control, six LBN). The rats were tested for ORM at 10 months of age. Both measures of this memory, the novel:familiar ratio (Fig. 1b) and the discrimination index (Fig. 1c) yielded similar results. Planned testing for an overall effect of pump found none (ratio; (F(1,13) = 0.24, p = 0.63), DI; (F(1,13) = 0.25, p = 0.62). A decrease in memory following LBN was apparent (ratio; (F(1,13) = 12.84, p = 0.003), DI; (F(1,13) = 17.66, p = 0.001) with no significant interaction (ratio; (F(1,13) = 0.03, p = 0.88), DI; (F(1,13) = 0.57, p = 0.46). This preparatory experiment indicated that the potential stress of surgery and of carrying a minipump in the adult did not impact memory assessed months later, during middle age. Therefore, subsequent experiments included the minipump/vehicle group as controls.
Then, to determine the effects of blocking CRHR1 in adulthood on the LBN-associated deficits in learning and memory, the CRHR1 blocker was infused to young LBN and control adults, and they were tested for ORM and OLM at 4, 8, and 12 months of age.
Object recognition memory (ORM)
Training parameters
In the training sessions, no animal had a discrimination index score indicating object preference (above 21), and all rats were included in the analysis. Similarly, during training, there were no overall effects of age (F(1.79, 25.02) = 1.15, p = 0.33), LBN (F(1, 14) = 1.56, p = 0.23), or antagonist (F(1, 14) = 1.34, p = 0.27) on the DI. In addition, early adversity did not alter ORM training across age (F(2, 28) = 0.37, p = 0.69) or with the CRHR1 blocker (F(1, 14) = 0.10, p = 0.76), and the effect of the blocker was not altered over time (F(2, 28) = 1.10, p = 0.35). Finally, interaction of age x LBN x antagonist was not observed (F(2, 28) = 1.81, p = 0.18). Altogether, these data excluded effects of either LBN or the CRHR1 antagonist on training in the ORM test.
Early-life adversity and CRHR1 blocker effects on object recognition memory
LBN affected ORM significantly, consistent with our prior work, and this effect increased with age (F(2, 28) = 3.51, p = 0.04). There were no overall effects of age (F(1.65, 23.17) = 0.75, p = 0.46), of the early-life adversity (F(1, 14) = 2.19, p = 0.16) or of the CRHR1 antagonist (F(1, 14) = 2.06, p = 0.17) (Fig. 2b, c)), Notably, neither age (F(2, 28) = 0.87, p = 0.43), nor LBN (F(1, 14) = 2.58, p = 0.13) influenced the effect of the antagonist (Fig. 2b, c). Additionally, there was no significant interaction between age x LBN x antagonist (F(2, 28) = 0.47, p = 0.63) (Fig. 2b, c). Given that the effect of the adverse early-life rearing was age dependent, we conducted post-hoc testing to inquire about potential overall age-related effects of the antagonist. We found no effect of the blocker on controls at 4 (p = 0.98), 8 (p = 0.98) or 12 (p = 0.98) months (Fig. 2b, c). In LBN-experiencing rats, adult administration of CRHR1 antagonist had no significant effects on object recognition at age 4 months (p = 0.98) or 8 months (p = 0.08). Surprisingly, the blocker significantly improved in object discrimination at 12 months of age (p = 0.05) (Fig. 2b, c). Thus, the CRHR1 blocker mitigated the age-related vulnerability of recognition memory engendered by early-life adversity [15, 24].
Total time investigating the objects was analyzed to exclude potential confounding by age or LBN (Supplementary Fig. 1a). There was an overall effect of age on the time investigating the objects during the testing phase (F(1.96, 27.42) = 20.45, p < 0.0001) with exploration times decreasing with age (Supplementary Fig. 1a). However, there were no effects of LBN (F(1, 14) = 0.10, p = 0.14) or the CRHR1 antagonist (F(1, 14) = 0.95, p = 0.34) (Supplementary Fig. 1a). No age x LBN (F(2, 28) = 0.15, p = 0.86) or time x antagonist (F(2, 28) = 0.65, p = 0.53) LBN x antagonist (F(1, 14) = 0.36, p = 0.56) or age x LBN x antagonist (F(2, 28) = 0.02, p = 0.98) interactions were identified, indicating that age effected total investigation time equally between groups (Supplementary Fig 1a).
Object location memory (OLM)
Training parameters
In the training sessions, no animal exhibited a significant object preference and all animals were included in the analyses. There were no main effects of age (F(1.92, 26.93) = 0.61, p = 0.55), LBN (F(1, 14) = 1.40, p = 0.26) or the CRHR1 antagonist (F(1, 14) = 1.75, p = 0.21) on DI during training. Neither early adversity (F(2, 28) = 0.27, p = 0.77) nor the CRHR1 antagonist (F(2, 28) = 0.92, p = 0.41) altered exploration time with age. The CRHR1 antagonist did not change object exploration differently across rearing conditions (F(1, 14) = 1.39, p = 0.26), nor did this change with age (F(2, 28) = 0.62, p = 0.55), Thus, age, LBN or the administration of a CRHR1 blocker did not affect training on the OLM task.
Early-life adversity and CRHR1 blocker effects on object location memory
LBN impaired location memory (F(1, 14) = 24.38, p = 0.0002), indicating a significant impairment of spatial memory, at all age groups following LBN (Fig. 2e, f), in line with our prior reports [24, 25]. There were no overall effects of age (F(1.59, 22.27) = 0.92, p = 0.39) or the CRHR1 blocker (F(1, 14) = 3.06, p = 0.10) (Fig. 2e, f). In addition, we did not identify interactions of age with LBN (F(2, 28) = 0.19, p = 0.83) or with the CRHR1 antagonist (F(2, 28) = 0.96, p = 0.39), nor was there an age x LBN x antagonist interaction (F(2, 28) = 0.35, p = 0.71) (Fig. 2e, f). Notably, the effect of the CRHR1 blocker depended on early-life adversity (F(1, 14) = 9.543, p = 0.008) (Fig. 2e, f). Planned post-hoc tests to determine antagonist effects found no differences in controls at 4 (p = 0.49), 8 (p = 0.49) or 12 (p = 0.49) months, nor significant differences at 4 (p = 0.30) or 8 (p = 0.49) months in the LBN group. Remarkably, the CRHR1 blocker rescued spatial memory at 12 months of age (p = 0.05) (Fig. 2e, f).
Age influenced total exploration time during testing (F(1.84, 25.82) = 20.84, p < 0.0001), as identified for the ORM task; however, there were no main effects of LBN (F(1, 14) = 0.43, p = 0.52) nor the CRHR1 antagonist (F(1, 14) = 1.03, p = 0.33) on this parameter (Supplementary Fig. 1b). Similarly, we did not observe significant age x LBN (F(2, 28) = 0.64, p = 0.53), age x CRHR1 antagonist (F(2, 28) = 0.57, p = 0.57), LBN x CRHR1 antagonist (F(1, 14) = 0.36, p = 0.55) or age x LBN x CRHR1 antagonist (F(2, 28) = 0.37, p = 0.70) interactions (Supplementary Fig. 1b)
Altogether, the findings indicate that hippocampus-dependent spatial memory is enduringly impaired by early-life adversity already at 4 months. Surprisingly, whereas prior CRHR1 blocker administration (at 2 months) does not prevent the deficits at 4 and 8 months, it mitigates these memory defects by middle age (12 months).
Early-life adversity and CRHR1 antagonist administered in early adulthood have no effects on anxiety- and depression-like behaviors in male rats
To define the scope of early-life adversity consequences and in view of the important contribution of CRH to stress-related behaviors, including anxiety and depression, we tested the rats in tasks that aim to measure anxiety and depression-like behaviors in rodents.
During the test for anxiety-like phenotypes on the elevated plus maze, there was an overall effect of age on the proportion (%) of time spent in the open arm (F(1.28, 17.33) = 4.75, p = 0.04) (Fig. 3a, b). There were no main effects of rearing in the LBN cages (F(1, 14) = 0.00, p = 0.94), consistent with prior work [59]. The CRHR1 blocker did not significantly influence the results (F(1, 14) = 3.96, p = 0.07) (Fig. 3a, b). We found no significant interactions of age x LBN (F(2, 27) = 2.41, p = 0.11), age x CRHR1 antagonist (F(2, 27) = 0.18, p = 0.83), LBN x CRHR1 antagonist (F(1, 14) = 0.12, p = 0.74), or age x LBN x CRHR1 antagonist (F(2, 27) = 0.72, 0.50) (Fig. 3a, b).
Similarly, there was an overall effect of age on the number of entries into the open arm of the maze (F(1.36, 27.94) = 4.239, p = 0.04) with no main effects of LBN (F(1, 41) = 3.41, p = 0.07), or the CRHR1 antagonist (F(1, 41) = 1.21, p = 0.28) (Supplementary Fig. 1c). Interactions of age x LBN (F(2, 41) = 0.28, p = 0.75), age x CRHR1 antagonist (F(2, 41) = 0.31, p = 0.73), LBN x antagonist (F(1, 41) = 0.07, p = 0.80), or age x LBN x antagonist (F(2, 41) = 2.54, p = 0.09) were also insignificant (Supplementary Fig. 1c). Thus, whereas age or the repeating of the tests several months apart seemed to decrease open arm entries and durations, the effect was consistent between the groups. In summary, neither the early-life adversity nor blocking of the CRH receptor within the brain influenced anxiety-like behaviors in this cohort of male rats.
Testing for depression-like behaviors in the Porsolt forced swim test identified no differences among the groups. Specifically, there were no main effects of age (F(1.28, 17.28) = 1.82, p = 0.19), LBN (F(1, 14) = 0.05, p = 0.83) or the CRHR1 blocker (F(1, 14) = 0.84, p = 0.38) on total time immobile in the forced swim test (Fig. 3c, d). Additionally, there were no age x LBN (F(2, 27) = 1.90, p = 0.17), age x antagonist (F(2, 27) = 1.65, p = 0.21), LBN x CRHR1 antagonist (F(1, 14) = 0.06, p = 0.82) or age x LBN x CRHR1 antagonist (F(2, 27) = 1.79, p = 0.19) interactions (Fig. 3c, d). Altogether the data support a lack of effect of early-life adversity or CRH receptor block on depression-related phenotypes.
Discussion
The principal findings of the experiments presented here are: (1) Early-life adversity provokes progressive deficits in both spatial and object memories, with earlier onset of the hippocampus-dependent memory deficits. (2) Mechanism-based interventions, even when administered in the adult, may ameliorate these memory problems in a modality and age-dependent manner. (3) CRH, acting within the brain, contributes to early-life adversity-induced memory problems and can be used to ameliorate them.
Early-life adversity provokes memory vulnerability that is more prominent for spatial memory and progresses to frank deficits with age
A broad literature now supports the emergence of deficits in learning and memory following early-life adversity [15, 17, 25, 50, 51]. Previous work has indicated that LBN impacts differentially spatial and non-spatial memory [25]. The ability to discriminate new objects in the testing phase of the ORM task utilizes multiple brain regions, including the hippocampus and the perirhinal cortex [60], whereas discrimination in OLM task is considered largely hippocampus dependent [61, 62]. We have previously discovered that spatial memory on the OLM task was impaired following early-life adversity as early as 2 months, while the ability to perform the ORM task was intact until 12 months of age [24, 25]. However, the apparently intact object memory masked incipient vulnerabilities: LBN-experiencing rats (but not those reared in control conditions) failed to recognize objects when exposed to a second stress during early adult life [25]. This vulnerability to both spatial and object memories was also unmasked with ageing [15]: 12-month-old LBN rats performed more poorly than controls in both prior studies [15] and in the current work. Indeed, early adversity may accelerate the impact of age on memory [15, 63].
Even in adulthood, a transient block of CRH receptors in hippocampus ameliorates memory problems provoked by early-life adversity
We have previously demonstrated that both systemic and intracerebral administration of a CRHR1 blocker immediately following a period of early-life adversity, significantly mitigated the spatial memory deficits provoked by early-life adversity. In that study, both the early-life adversity and the CRHR1 block were carried out during the first weeks of life, an apparent sensitive period for hippocampal dendritic arborization, synaptic maturation, and memory formation [11, 28, 64, 65].
Indeed, the developing hippocampus is more sensitive to stress, and specifically to molecules unleashed by stress/adversity. Direct effects of glucocorticoids, arriving from the adrenal during early-life stress, on dendritic arborization in hippocampal neurons have been demonstrated [34]. Similarly, stress levels of CRH lead to loss of synapses and dendritic spines [31, 44]. Chronic exposure to CRH stunts dendritic arborization of developing neurons [28] in rodents, and potentially in humans [66]. Thus, a putative mechanism for the enduring memory problems provoked by early-life adversity is an irreversible loss of synapses and of synapse-carrying dendrites [16, 24], via concerted actions of glucocorticoids and CRH [67]. The excitotoxic actions of glucocorticoids on dendrites are well documented [29, 68]. Notably, CRH at stress levels, excites neurons [41, 69] and can destroy dendritic spines and synapses via an NMDA-receptor-mediated process [46]. The progressive nature of the memory problems provoked by early-life adversity may derive from the cumulative effects of additional chronic or recurrent spine- and dendritic damage sustained by already compromised neurons when recurrent minor stresses occur during life and promote release of glucocorticoids and local hippocampal CRH.
Administration of a CRH receptor blocker during the vulnerable developmental period should prevent the initial neuronal injury that predisposes to further loss of synapses and dendrites. Therefore, it is unsurprising that blocking CRHR1 at P10 can rescue memory quite completely [24].
In the present study, the blocking of CRHR1 was performed in the adult brain. As many of the morbidities associated with early-life adversity do not emerge until adulthood, it is difficult to determine whether interventions will be required. Therefore, it is vital to know if interventions given around the onset of symptoms may still be effective. Surprisingly, deficits on ORM that occur in LBN animals at 12 months of age were rescued by administration of the CRHR1 antagonist (Fig. 2b), and a similarly age-dependent effect was found at 12 months in the OLM task (Fig. 2e). These striking findings suggest that interventions later in life can ameliorate progressive memory loss and raise two crucial questions: First, how might CRH blockade for a transient period in the adult work, and second, why is the memory rescue more prominent during middle age?
How might transient block of hippocampal CRHR1 in the adult rescue memory from the impact of early-life adversity?
As mentioned above, acute increases in hippocampal CRH release in the adult hippocampus occur during stress [70]. These stress levels of CRH by themselves as well as in concert with corticosterone, destroy dendritic spines and synapses [44, 67], by disrupting the actin cytoskeleton of spines [31]. Hippocampi of LBN-experiencing adult rats have increases in CRH-positive interneurons and increased CRH mRNA expression (Fig. 4) [24, 71]. This is accompanied by a decrease in dendritic branching [15, 25]. The LBN adult hippocampus is both rich in CRH, which is released upon stress during adult life, and consists of compromised neurons with stunted dendritic arborization. We propose that a transient (1-week) block of CRHR1 allows neurons to recover, potentially providing them with resilience for the subsequent impact of life-long stresses. This notion is consistent with the finding that the effect of the CRHR1 blocker was most pronounced at 12 months, when cumulative age-dependent injuries to hippocampal neurons would be maximal.
Proposed mechanism for effect of CRHR1 blocker when given to young adult rats. Following early adversity there is an increase in CRH-positive interneurons (IN) within the hippocampus, which is associated with increases in CRH mRNA and subsequently elevated levels of CRHR1 at the synapse on pyramidal cells (PC). In early-life adversity, this causes reductions of synapses and memory impairments in later life. Blocking CRHR1 with the antagonist for a week in the adult brain, may cause a decrease in CRH binding, which decreases receptor expression at the synapse. By reducing the number of CRH receptors, spines may be less sensitive to increases in CRH thereby making them less prone to collapse and maintaining memory processing over time
There is evidence for an interplay between the levels of CRH and CRHR1, with elevated hippocampal CRH levels being associated with increases in CRH receptor mRNA [72] (Fig. 4). We can then speculate that blocking CRHR1 for a week in the adult brain may cause a decrease in CRH binding, which in turn is sufficient to decrease receptor expression at the synapse (Fig. 4). By reducing the number of CRH receptors, spines may be less sensitive to increases in CRH thereby making them less prone to collapse and maintaining memory processing over time (Fig. 4).
The transient blockade of CRHR1 during adulthood may also persistently repress CRH expression levels, as shown previously for the same intervention early in life [24]. This might take place by counteracting the corrupted epigenetic/transcriptomic regulatory processes in the hippocampus of the adversity-experiencing rats, which lead to persistent upregulation of hippocampal CRH. CRH expression is potentially regulated by the transcription factors GR and the repressor neuron-restrictive silencer factor (NRSF) [18, 73]. Both GR and NRSF are dysregulated in hippocampi of adversity-experiencing rats [18]. Specifically, gene set enrichment analyses demonstrate that gene targets of NRSF and GR, including those involved in dendritic growth and synaptic maturation are repressed, potentially accounting for altered cellular properties and maturation of hippocampal neurons and circuits. Our transient interference with CRHR1-CRH regulatory loops may reset upstream transcriptional processes regulating CRH expression itself. Future studies will aim to examine these potential mechanisms via transcriptomic neuroanatomic and physiological approaches.
Limitations and caveats
While the studies presented here provide convincing evidence that interventions given in adulthood mitigate memory deficits following early-life adversity, there are additional considerations. In the present study, we administered the antagonist via ICV rather than directly into the hippocampus. While this administration might have elicited effects from structures outside of the hippocampus, this administration mode prevents the need for infusions into the hippocampus, requiring bilateral surgeries for both anterior and posterior hippocampus and increasing the chances of damage to the hippocampus itself. In addition, translational studies would likely involve systemic administration of the CRHR1 blocker.
In addition, it is possible that the ICV infusion of the CRHR1 blocker influenced CRH receptors in the pituitary and attenuated the overall stress responses for a week. This is unlikely, as we have previously infused similar and higher doses of the antagonist and demonstrated that they do not leave the brain, and do not influence stress-induced spikes of plasma corticosterone [74].
The longitudinal approach adopted here allows for analysis of effects within subjects, across the lifespan, however, this requires repeat testing on tasks. This approach has been previously validated for the learning and memory tasks by ensuring long intervals (longer than 2 months) between tests. In addition, modifications such as using different objects minimize potential confounders [62]. Notably, any confounding factors, such as those noted for repeated anxiety tests, would be consistent between all testing groups. In addition, we recognize that group sizes in this study are modest. The observed effect sizes of the CRHR1 blocker on memory improvement were robust and conclusive. However, the current group sizes may not enable excluding subtle effects of the antagonist on control animals or on anxiety-related behaviors.
Does early-life adversity provoke aberrant emotional-like behaviors in rodents?
The effects of early-life adversity on measures of emotional function in rodents are diverse, and highly species and sex-dependent [52, 75, 76]. In male rats in the present study (Fig. 3) and our prior work [15, 59], we have found no effect on EPM or FST [15, 50, 59, 77]. However, increased anxiety-like phenotypes [76, 78, 79] and increased immobility time during FST [80] have been described by others following the LBN paradigm. Notably, we have identified serious defects in the emotional reward circuit after early-life adversity the emergence of severe anhedonia-like behavior [59, 77, 81]. This was not tested in the current work, so that future studies will explore if blocking CRHR1 within the brain or within targeted nodes of the reward circuitry might ameliorate the anhedonia, a trans-diagnostic entity with implication for risk taking, drug use, and depression in humans.
Conclusion
In summary, we show here that early adversity causes distinct types of memory deficits, which worsen with age. Post-hoc mechanism-based interventions in the adult significantly mitigate these problems in an age and task-specific manner, offering hope for the development of therapies to the large proportion of individuals who grow up in adverse circumstances around the world.
References
Prince M, Wimo A, Guerchet M, Gemma-Claire Ali M, Wu Y-T, Prina M, et al. World Alzheimer Report 2015. The global impact of dementia an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015.
Prince M, Guerchet M, Prina M. Policy brief for heads of government: the global impact of dementia. London: Alzheimer's Disease International; 2013. p. 2013–50.
Klengel T, Binder EB. Epigenetics of stress-related psychiatric disorders and gene × environment interactions. Neuron. 2015;86:1343–57.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9.
Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944-45. Br J Psychiatry. 1995;166:601–6.
Eriksson M, Räikkönen K, Eriksson JG. Early life stress and later health outcomes-findings from the Helsinki Birth Cohort Study. Am J Hum Biol. 2014;26:111–6.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Chen Y, Baram TZ. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41:197–206.
Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, Tyrka AR. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103.
Raymond C, Marin M-F, Majeur D, Lupien S. Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:152–60.
Avishai-Eliner S. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–24.
Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala E-LL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–63.
Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–40.
Short AK, Baram TZ. Adverse early-life experiences and neurologic disease: age-old questions and novel answers. Nat Rev Neurol. 2019;2019:657–69.
Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–38.
Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–24.
Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. 2016;82:64–71.
Schulmann A, Bolton JL, Curran MM, Regev L, Kamei N, Singh-Taylor A, et al. Blocking NRSF function rescues spatial memory impaired by early-life adversity and reveals unexpected underlying transcriptional programs. SSRN Electron J. 2019. Nov. https://doi.org/10.2139/ssrn.3284454.
Singh-Taylor A, Korosi A, Molet J, Gunn BG, Baram TZ. Synaptic rewiring of stress-sensitive neurons by early-life experience: A mechanism for resilience? Neurobiol Stress. 2015;1:109–15.
Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16:332–44.
Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, Thomas KM, et al. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–9.
Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66.
Hatfield T, Wing DA, Buss C, Head K, Muftuler LT, Davis E. 71: Magnetic resonance imaging (MRI) shows long term changes in brain structure in preterm infants exposed to chorioamnionitis. Am J Obstet Gynecol. 2011;204:S41.
Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–15.
Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, et al. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016;26:1618–32.
Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, et al. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci. 2013;16:706–13.
Wang X-D, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis. 2011;42:300–10.
Curran MM, Sandman CA, Poggi Davis E, Glynn LM, Baram TZ. Abnormal dendritic maturation of developing cortical neurons exposed to corticotropin releasing hormone (CRH): insights into effects of prenatal adversity? PLoS ONE. 2017;12:e0180311.
Magarinõs AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995;69:83–8.
Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66.
Chen Y, Kramár EA, Chen LY, Babayan AH, Andres AL, Gall CM, et al. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry. 2013;18:485–96.
Zhou Q, Homma KJ, Poo M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–57.
Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–84.
Alfarez DN, De Simoni A, Velzing EH, Bracey E, Joëls M, Edwards FA, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19:828–36.
Yan X-X, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: Light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–43.
Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–81.
Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci. 2004;101:15782–7.
Hooper A, Maguire J. Characterization of a novel subtype of hippocampal interneurons that express corticotropin-releasing hormone. Hippocampus. 2016;26:41–53.
Hooper A, Fuller PM, Maguire J. Hippocampal corticotropin-releasing hormone neurons support recognition memory and modulate hippocampal excitability. PLoS ONE. 2018;13:e0191363.
Gunn BG, Sanchez GA, Lynch G, Baram TZ, Chen Y. Hyper-diversity of CRH interneurons in mouse hippocampus. Brain Struct Funct. 2019;224:583–98.
Gunn BG, Cox CD, Chen Y, Frotscher M, Gall CM, Baram TZ, et al. The endogenous stress hormone CRH modulates excitatory transmission and network physiology in hippocampus. Cereb Cortex. 2017;27:4182–98.
Chen Y, Brunson KL, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–23.
Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–7.
Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–11.
Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA. 2010;107:13123–8.
Andres AL, Regev L, Phi L, Seese RR, Chen Y, Gall CM, et al. NMDA receptor activation and calpain contribute to disruption of dendritic spines by the stress neuropeptide CRH. J Neurosci. 2013;33:16945–60.
Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453.
McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22.
Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758.
Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–28.
Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–900.
Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 2017;20:421–48.
Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–61.
Levis SC, Bentzley BS, Bolton JL, Molet J, Baram TZ, Mahler SV. On the origins of a selective vulnerability to opioid addiction. bioRxiv. 2019:716522.
Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014;56:1675–88.
Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–9.
Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton JL, Noam Y, et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol Psychiatry. 2018;23:648–57.
Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69.
Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702–2.
Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–70.
Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57.
Vogel-Ciernia A, Wood MA. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. 2014;69:8.31.1–17.
Yajima H, Haijima A, Khairinisa MA, Shimokawa N, Amano I, Takatsuru Y. Early-life stress induces cognitive disorder in middle-aged mice. Neurobiol Aging. 2018;64:139–46.
Travaglia A, Bisaz R, Cruz E, Alberini CM. Developmental changes in plasticity, synaptic, glia and connectivity protein levels in rat dorsal hippocampus. Neurobiol Learn Mem. 2016;135:125–38.
Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1973;63:195–204.
Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, Baram TZ. Cortical thinning and neuropsychiatric outcomes in children exposed to prenatal adversity: a role for placental CRH? Am J Psychiatry. 2018;175:471–9.
Chen Y, Molet J, Lauterborn JC, Trieu BH, Bolton JL, Patterson KP, et al. Converging, synergistic actions of multiple stress hormones mediate enduring memory impairments after acute simultaneous stresses. J Neurosci. 2016;36:11295–307.
McEwen BS, Cameron H, Chao HM, Gould E, Magarinos AM, Watanabe Y, et al. Adrenal steroids and plasticity of hippocampal neurons: toward an understanding of underlying cellular and molecular mechanisms. Cell Mol Neurobiol. 1993;13:457–82.
Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–9.
Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–40.
Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front Neuroendocrinol. 2006;27:180–92.
Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol. 2002;176:75–86.
McClelland S, Brennan GP, Dubé C, Rajpara S, Iyer S, Richichi C, et al. The transcription factor NRSF contributes to epileptogenesis by selective repression of a subset of target genes. Elife. 2014;3:e01267.
Chen Y, Fenoglio KA, Dubé CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry. 2006;11:992–1002.
Manzano Nieves G, Schilit Nitenson A, Lee H-I, Gallo M, Aguilar Z, Johnsen A, et al. Early life stress delays sexual maturation in female mice. Front Mol Neurosci. 2019;12:27.
Guadagno A, Wong TP, Walker C-D. Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Prog Neuro-Psychopharmacology Biol Psychiatry. 2018;81:25–37.
Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, et al. Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol Psychiatry. 2018;83:137–47.
Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segala S, Salum GA, et al. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry. 2012;2:e195.
Wang X-D, Labermaier C, Holsboer F, Wurst W, Deussing JM, Müller MB, et al. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci. 2012;36:2360–7.
Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–65.
Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, et al. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress. 2018;8:57–67.
Funding and disclosure
The authors declare no competing interests. Work was supported by NIH grants NS28912, MH73136, and MH096889.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Short, A.K., Maras, P.M., Pham, A.L. et al. Blocking CRH receptors in adults mitigates age-related memory impairments provoked by early-life adversity. Neuropsychopharmacol. 45, 515–523 (2020). https://doi.org/10.1038/s41386-019-0562-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0562-x
This article is cited by
-
Early life stress induces age-dependent epigenetic changes in p11 gene expression in male mice
Scientific Reports (2021)