Abstract
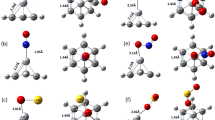
Ab initio calculations are performed to study hydrogen storage properties of Ti-doped benzene and Ti-doped nitrogen-substituted benzene complexes. Two of the carbon atoms in benzene are replaced by two nitrogen atoms. Two nitrogen atoms are substituted either at 1-2, 1-3, or 1-4 positions of a benzene ring and named as BN1-2Ti, BN1-3Ti, and BN1-4Ti, respectively. Maximum four, four, three, and four H2 molecules get adsorbed on C6H6Ti, BN1-2Ti, BN1-3Ti, and BN1-4Ti complexes respectively with respective H2 uptake capacity of 6.02, 5.84, 4.45, and 5.84 wt%. The positive Gibbs free energy corrected H2 adsorption energy values obtained for all these complexes at ambient conditions indicate that the formation of these complexes at room temperature is thermodynamically favorable. Temperature- and pressure-dependent adsorption energy calculations show that the H2 adsorption on all these complexes is feasible over a wide range of temperature and pressure. The gap between the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbital (LUMO) is found to be greater than 5 eV for all the complexes indicating stability of these complexes. The H2 molecules interact more strongly with Ti-doped nitrogen-substituted benzene than the Ti-doped benzene that results in higher H2 desorption temperature obtained using van 't Hoff equation for the former than the latter. The density of states plots have been used to understand the H2 adsorption mechanism.






Similar content being viewed by others
References
Jena P (2011). J Phys Chem Lett 2:206
Huang B, Lee H, Duan W, Ihm J (2008). Appl Phys Lett 93:063107
Kolmann SJ, Chan B, Jordan MJT (2008). Chem Phys Lett 467:126
Lee H, Ihm J, Cohen ML, Louie SG (2010). Nano Lett 10:793
Anafcheh M, Naderi F (2018). Int J Hydrog Energy 43:12271
Yildirim T, Iniguez J, Ciraci S (2005). Phys Rev B 72:153403
Kalamse V, Wadnerkar N, Deshmukh A, Chaudhari A (2012). Int J Hydrog Energy 37:5114
Mei F, Ma X, Bie Y, Xu G (2017). J Comp Chem 16:1750065
Weck PF, Dhilip Kumar TJ, Kim E, Balakrishnan N (2007). J Chem Phys 126:094703
Dong LX, Hong Z, Jian TY, Dong WW, Yang WC (2012). Chin J Struct Chem 31:459
Kalamse V, Wadnerkar N, Chaudhari A (2013). Energy 49:469
Phillips AB, Shivaram BS, Myneni GR (2012). Int J Hydrog Energy 37:1546
Sun Q, Wang Q, Jena P, Kawazoe Y (2005). J Am Chem Soc 127:14582
Durgun E, Ciraci S, Yildirim T (2008). Phys Rev B 77:085405
Yuan L, Chen Y, Kang L, Zhang C, Wang D, Wang C, Zhang M, Wu X (2017). App Sur Sci 399:463
Huang X, Zhao YJ, Liao JH, Yang XB (2016). Int J Hydrog Energy 41:11275
Deshmukh A, Konda R, Kalamse V, Chaudhari A (2016). RSC Adv 6:47033
Tavhare P, Titus E, Chaudhari A (2018). Int J Hydrog Energy 44:345
Tavhare P, Deshmukh A, Chaudhari A (2017). Phys Chem Chem Phys 19:681
Lin IH, Tong YJ, Hsieh HJ, Huang HW, Chen HT (2016). Int J Energy Res 40:230
Huang HW, Hsieh HJ, Lin IH, Tong YJ, Chen HT (2015). J Phys Chem C 119:7662
Sankaran M, Viswanathan B (2006). Carbon 44:2816
Wang L, Yang FH, Yang RT (2009). AIChE J 55:1823
He H, Chen X, Zou W, Li R (2018). Int J Hydrog Energy 43:2823
Wang L, Yang RT (2009). J Phys Chem C 113:21883
Omidvar A (2017). Chem Phys 493:85
Srinivasu K, Ghosh SK (2012). J Phys Chem C 116:25184
Srivastava AK, Misra N (2015). Chem Phys Lett 625:5
Ewels CP, Glerup M (2005). J Nanosci Nanotechnol 5:1345
Ayala P, Arenal R, Rummeli M, Rubio A, Pichler T (2010). Carbon 48:575
Møller C, Plesset MS (1934). Phys Rev 46:618
O’Boyle NM, Tenderholt AL, Langner KM (2008). J Comput Chem 29:839
Ma LJ, Jia J, Wu HS (2015). Chem Phys 457:57
Lide DR (1994) CRC handbook of chemistry and physics75th edn. CRC Press, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford CT
Acknowledgments
Financial support to Priyanka Tavahre from Department of Science and Technology, India under Womens Scientist Scheme - A (Grand No: SR/WOS-A/PM-33/2017) is thankfully acknowledged. Thanks to The Institute of Science, Mumbai. Bioinformatics Resources and Applications Facility (BRAF) from C-DAC, Pune is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The work has not been submitted elsewhere for publication. The claimed new results express our own findings.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tavhare, P., Chaudhari, A. Nitrogen substitution effect on hydrogen adsorption properties of Ti-decorated benzene. Struct Chem 30, 2151–2158 (2019). https://doi.org/10.1007/s11224-019-01340-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01340-x