Abstract
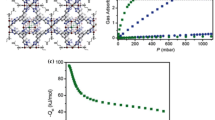
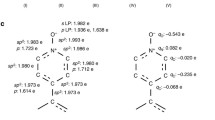
The present work is focused in the theoretical study of a porous coordination polymer formed by isonicotinylhydrazine (INH), 1,4-benzenedicarboxylic (14BDC) acid, and Co2+. This coordination polymer [Co{CP}] was studied by density of states and charge density difference, which allowed determining its electronic properties, transitions of valence to conduction band and its post-synthesis capacity. We have also evaluated the possibility of inserting lithium into the structure and observed that the resulting structure is stable and energetically favorable. Furthermore, taking into account gas adsorption applications, the insertion of a H2 was also conducted in both [Co{CP}] and lithium-doped structure. Our results have showed that the adsorption of hydrogen in Co{CP} is energetically unfavorable, while in the lithium-doped structure, it showed remarkable potential. Overall, we were able to show in this work after thorough calculation that the insertion of lithium ions into the coordination polymer structure is highly beneficial, improving drastically its hydrogen adsorption ability, which opens a wide window of opportunities towards the development of new coordination polymers for gas adsorption.









Similar content being viewed by others
References
Batten SR, Champness NR, Chen XM, Garcia-Martinez J, Kitagawa S, Ohrstrom L, O'Keeffe M, Suh MP, Reedijk J (2013) Terminology of metal-organic frameworks and coordination polymers (IUPAC recommendations 2013). Pure Appl Chem 85(8):1715–1724. https://doi.org/10.1351/pac-rec-12-11-20
Steed JW, Atwood JL (2009) Supramolecular chemistry2nd edn. John Wiley and Son Ltd, West Sussex
Millward AR, Yaghi OM (2005) Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J Am Chem Soc 127(51):17998–17999. https://doi.org/10.1021/ja0570032
Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K (2000) A homochiralmetal-organic porous material for enantioselective separation and catalysis. Nature 404
Matsuda R, Kitaura R, Kitagawa S, Kubota Y, Belosludov RV, Kobayashi TC, Sakamoto H, Chiba T, Takata M, Kawazoe Y, Mita Y (2005) Highly controlled acetylene accommodation in a metal-organic microporous material. Nature 436(7048):238–241. https://doi.org/10.1038/nature03852
Chen F, Wang Y, Bai D, He M, Gao X, He Y (2018) Selective adsorption of C2H2 and CO2 from CH4 in an isoreticular series of MOFs constructed from unsymmetrical diisophthalate linkers and the effect of alkoxy group functionalization on gas adsorption. J Mater Chem A 6(8):3471–3478. https://doi.org/10.1039/c7ta10123f
Sun CY, Qin C, Wang CG, Su ZM, Wang S, Wang XL, Yang GS, Shao KZ, Lan YQ, Wang EB (2011) Chiral nanoporous metal-organic frameworks with high porosity as materials for drug delivery. Adv Mater 23(47):5629. https://doi.org/10.1002/adma.201102538
Combelles C, Ben Yahia M, Pedesseau L, Doublet ML (2011) FeII/FeIII mixed-valence state induced by Li-insertion into the metal-organic-framework Mil53(Fe): a DFT+U study. J Power Sources 196(7):3426–3432. https://doi.org/10.1016/j.jpowsour.2010.08.065
Maark TA, Pal S (2010) A model study of effect of M = Li+, Na+, Be2+, Mg2+, and Al3+ ion decoration on hydrogen adsorption of metal-organic framework-5. Int J Hydrog Energy 35(23):12846–12857. https://doi.org/10.1016/j.ijhydene.2010.08.054
Dalach P, Frost H, Snurr RQ, Ellis DE (2008) Enhanced hydrogen uptake and the electronic structure of lithium-doped metal−organic frameworks. J Phys Chem C 112(25):9278–9284. https://doi.org/10.1021/jp801008d
Kolmann SJ, Chan B, Jordan MJT (2008) Modelling the interaction of molecular hydrogen with lithium-doped hydrogen storage materials. Chem Phys Lett 467(1–3):126–130. https://doi.org/10.1016/j.cplett.2008.10.081
Rao D, Lu R, Xiao C, Kan E, Deng K (2011) Lithium-doped MOF impregnated with lithium-coated fullerenes: a hydrogen storage route for high gravimetric and volumetric uptakes at ambient temperatures. Chem Commun 47(27):7698–7700. https://doi.org/10.1039/c1cc11832c
Morozan A, Jaouen F (2012) Metal organic frameworks for electrochemical applications. Energy Environ Sci 5(11):9269–9290. https://doi.org/10.1039/c2ee22989g
Jiang ZJ, Zhao PS, Li RQ, An LT, Zhang ZC, Xu J (2013) Two distinct Cu(I) coordination polymers based on isoniazid and different bridged groups. J Chem Crystallogr 43(9):463–470. https://doi.org/10.1007/s10870-013-0442-x
Xue H, Na Z, Wu Y, Wang X, Li Q, Liang F, Yin D, Wang L, Ming J (2018) Unique Co3O4/nitrogen-doped carbon nanospheres derived from metal–organic framework: insight into their superior lithium storage capabilities and electrochemical features in high-voltage batteries. J Mater Chem A 6(26):12466–12474. https://doi.org/10.1039/c8ta03959c
de Almeida FB, e Silva FH, Yoshida MI, de Abreu HA, Diniz R (2013) An interpenetrated 2D coordination polymer: a candidate for gas adsorption of small molecules. Inorg Chim Acta 402:60–68. https://doi.org/10.1016/j.ica.2013.03.038
Sheldrick G (2008) A short history of SHELX. Acta Crystallogr Sect A 64(1):112–122. https://doi.org/10.1107/S0108767307043930
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van De Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457. https://doi.org/10.1107/s002188980600731x
Barnes CL (1997) ORTEP-3 for Windows - a version of ORTEP-III with a graphical user interface (GUI). J Appl Crystallogr 30(1):568–568. https://doi.org/10.1107/s0021889897006638
Perdew JP, Zunger A (1981) Self-interaction correction to density-functional approximations for many-electron systems. Phys Rev B 23(10):5048–5079. https://doi.org/10.1103/PhysRevB.23.5048
Baroni S, dalCorso A, deGironcoli S, Giannozzi P, Cavazzoni C (2018) http://www.democritos.it. 2018
Laasonen K, Pasquarello A, Car R, Lee C, Vanderbilt D (1993) Car-Parrinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials. Phys Rev B 47(16):10142–10153. https://doi.org/10.1103/PhysRevB.47.10142
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev B 136(3B):B864. https://doi.org/10.1103/PhysRev.136.B864
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):1133
Payne MC, Teter MP, Allan DC, Arias TA, Joannopoulos JD (1992) Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients. Rev Mod Phys 64(4):1045–1097. https://doi.org/10.1103/RevModPhys.64.1045
Mehio N, Dai S, Jiang D-e (2014) Quantum mechanical basis for kinetic diameters of small gaseous molecules. J Phys Chem A 118(6):1150–1154. https://doi.org/10.1021/jp412588f
Isaeva VI, Kustov LM (2007) Metal-organic frameworks—new materials for hydrogen storage. Russ J Gen Chem 77(4):721–739. https://doi.org/10.1134/s1070363207040342
Bader RFW, Hernández-Trujillo J, Cortés-Guzmán F (2007) Chemical bonding: from Lewis to atoms in molecules. J Comput Chem 28(1):4–14. https://doi.org/10.1002/jcc.20528
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18(1):9–15. https://doi.org/10.1021/ar00109a003
Koch U, Popelier PLA (1995) Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem 99(24):9747–9754. https://doi.org/10.1021/j100024a016
Oliveira BG, Araújo RCMU (2010) A TOPOLOGIA MOLECULAR QTAIM E A DESCRIÇÃO MECÂNICO-QUÂNTICA DE LIGAÇÕES DE HIDROGÊNIO E LIGAÇÕES DE DI-HIDROGÊNIO. Quim Nova 33(5):1155–1162
Funding
The authors thank the Brazilian agency CAPES, CNPq (project number 474173/2013-5) and FAPEMIG for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2316 kb)
Rights and permissions
About this article
Cite this article
De Almeida, F.B., De Abreu, H.A. & Diniz, R. Theoretical calculations of a porous coordination polymer formed by isonicotinylhydrazine, 1,4-benzenedicarboxylic and Co2+: electronic properties, lithium doping, and H2 adsorption studies. Struct Chem 30, 2369–2377 (2019). https://doi.org/10.1007/s11224-019-01367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01367-0