Abstract
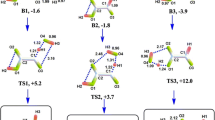
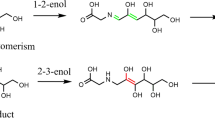
Thermodynamic and kinetic studies of the hydrogen atom transfer (HAT) from hydroxyl (OH) groups of four kaempferol-based compounds, namely kaempferol, morin, morin-5*-sulfonate and morin-7-O-sulfate to hydroxyl radical (·OH), have been carried out using density functional theory (DFT) methods at the CAM-B3LYP/6–311++G(d,p) level equipped with polarizable continuum model (PCM) of solvation. All HAT reactions in aqueous solution are exothermic and spontaneous. For most compounds, the most preferable OH group for HAT is situated at position C3 (O3-H3) on the pyrone ring. The reaction potential of such a reactive group is found to be highest in morin-7-O-sulfate. The rate constants for the HAT reactions at different OH groups of each compound have been determined based on the transition state theory. The presence of substituents leads to the variation in either the characteristic interactions at the reactive site or the charge distribution on transition-state geometries, hence significantly affecting the kinetics of HAT. The highest rate of HAT is resulted for the OH group at position C4* (O4*-H4*) on the phenyl ring (ring B) of morin-5*-sulfonate because a hydrogen bond between ·OH and the sulfonate group favors the formation of transition state. However, for most compounds under study, the HAT reaction at O3-H3 initiated by ·OH is highly favorable both thermodynamically and kinetically.







Similar content being viewed by others
References
Chen AY, Chen YC (2013). Food Chem 138:2099–2107
Alkhamees A (2013) O. Br J Pharmacol Toxicol 4:10–17
Shahabadi N, Mohammadpour M (2012). Spectrochim Acta A Mol Biomol Spectrosc 86:191–195
Pieniążek E, Kalembkiewicz J, Dranka M, Woźnicka E (2014). J Inorg Biochem 141:180–187
Chen Y, Zheng R, Jia Z, Ju Y (1990). Free Radic Biol Med 9:19–21
Amić D, Davidović-Amić D, Beslo D, Trinajstić N (2003). Croat Chem Acta 76:55–61
Treml J, Šmejkal K (2016). Compr Rev Food Sci Food Saf 15:720–738
Husain SR, Cillard J, Cillard P (1987). Phytochemistry 26:2489–2491
Chen J-W, Zhu Z-Q, Hu T-X, Zhu D-Y (2002). Acta Pharmacol Sin 23:667–672
Chen X, Deng Z, Zhang C, Zheng S, Pan Y, Wang H, Li H (2018). Food Res Int. https://doi.org/10.1016/j.foodres.2018.11.018
Dar RA, Naikoo GA, Hassan IU, Shaikh AMH (2016). Anal Chem Res 7:1–8
Li H-W, Zou T-B, Jia Q, Xia E-Q, Cao W-J, Liu W, He T-P, Wang Q (2016). Biomed Pharmacother 84:909–916
Doroshenko AO, Posokhov EA, Verezubova AA, Ptyagina LM (2000). J Phys Org Chem 13:253–265
Georgieva I, Trendafilova N, Aquino AJA, Lischka H (2006). J Phys Chem A 111:127–135
Marković Z, Milenković D, Đorović J, Dimitrić Marković JM, Stepanić V, Lučić B, Amić D (2012). Food Chem 135:2070–2077
Yasarawan N, Thipyapong K, Ruangpornvisuti V (2014). J Mol Graph Model 51:13–26
Fiorucci S, Golebiowski J, Cabrol-Bass D, Antonczak S (2004). Chem Phys Chem 5:1726–1733
Sadasivam K, Kumaresan R (2011). Mol Phys 109:839–852
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams DF, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 (Revision B.01). Gaussian Inc., Wallingford
Yanai T, Tew DP, Handy NC (2004). Chem Phys Lett 393:51–57
Jacquemin D, Perpète EA, Scuseria GE, Ciofini I, Adamo C (2008). J Chem Theory Comput 4:123–135
Shchavlev AE, Pankratov AN, Shalabay AV (2005). J Phys Chem A 109:4137–4148
Hao C, Tureček F (2009). J Am Soc Mass Spectrom 20:639–651
Li M, Xie L-F, Ju X-H, Zhao F-Q (2013). Petrol Chem+ 53:431–437
Yasarawan N, Thipyapong K, Ruangpornvisuti V (2016). J Mol Struct 1107:278–290
Marenich AV, Cramer CJ, Truhlar DG (2009). J Phys Chem B 113:6378–6396
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1998) NBO Version 3.1. TCI, University of Wisconsin, Madison
Zhao J, Zhang R (2008) In: Sabin J, Brandas E (eds) Advances in Quantum Chemistry: Applications of Theoretical Methods to Atmospheric Science, vol 55. Academic Press, San Diego, pp 177–214
Suwattanamala A, Ruangpornvisuti V (2009). Struct Chem 20:619–631
Seyoum A, Asres K, El-Fiky FK (2006). Phytochemistry 67:2058–2070
Matei I, Tablet C, Ionescu S, Hillebrand M (2014). Rev Roum Chim 59:401–405
Anouar EH, Marakchi K, Komiha N, Kabbaj OK, Dhaouadi Z, Lahmar S (2009). Phys Chem News 45:107–113
Atohoun YGS, Doco RC, Houngue MTAK, Kuevi AU, Kpotin GA, Mensah J-B (2016). Am J Sci Ind Res 7:145–152
Dimitrić Marković JM, Milenković D, Amić D, Popović-Bijelić A, Mojović M, Pašti IA, Marković ZS (2014). Struct Chem 25:1795–1804
van Acker SABE, de Groot MJ, van den Berg D-J, Tromp MNJL, Donne-Op den Kelder G, van der Vijgh WJF, Bast A (1996). Chem Res Toxicol 9:1305–1312
Rong YZ, Wang ZW, Zhao B (2013). Food Biophys 8:90–94
Bondi A (1964). J Phys Chem 68:441–452
Parthasarathi P, Subramanian V (2006) Characterization of Hydrogen Bonding: From van der Waals Interactions to Covalency. In: Grabowski SJ (ed) Hydrogen Bonding - New Insights, vol 3. Challenges and Advances in Computational Chemistry and Physics, vol 3. Springer, Dordrecht, pp 1–50
Cao S, Jiang X, Chen J (2010). J Inorg Biochem 104:146–152
Souza RFV, De Giovani WF (2005). Spectrochim Acta A Mol Biomol Spectrosc 61:1985–1990
Acknowledgements
We would like to thank Dr. Akapong Suwattanamala at Burapha University for his valuable advices.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This statement is to declare that there are no known conflicts of interest associated with the manuscript entitled “Exploring the transfer of hydrogen atom from kaempferol-based compounds to hydroxyl radical at ground state using PCM/DFT approach” by Khajadpai Thipyapong and Nuttawisit Yasarawan. There has been no significant financial support for this work that could have influenced its outcome. We further confirm that there are no ethical issues to declare in this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17682 kb)
Rights and permissions
About this article
Cite this article
Thipyapong, K., Yasarawan, N. Exploring the transfer of hydrogen atom from kaempferol-based compounds to hydroxyl radical at ground state using PCM-DFT approach. Struct Chem 30, 2167–2180 (2019). https://doi.org/10.1007/s11224-019-01331-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01331-y