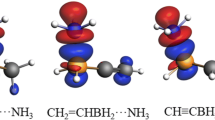
Abstract
In this paper, we argued that the classical explanation to the formation of trigonal planar coordination of the center N and O atoms in N(SiR3)3 and [O(SiR3)3]+ by forming 4-center 2-electron conjugated d-pπ bond was questionable, since it could not correctly predict the configurations of P(SiR3)3 and [S(SiR3)3]+. We found that the trigonal planar configuration of the center N and O atoms was strongly correlated to the greater difference between the electronic negativities of the center atom N/O, and the around Si atoms. We proposed that repulsive interactions among the three positive charged YR3 (Y=Si) moieties resulted in the trigonal planar configuration of the center N and O atoms. This proposal was supported by the predicted trigonal planar configurations of model species [F(BH3)3]− and [O(BH3)3]2−, in which the formation of 4-center 2-electron conjugated d-pπ bond was impossible.


Similar content being viewed by others
References
Becke AD (1993). J Chem Phys 98:5648–5652
Cooper DL (2002) Valence Bond Theory. Ed. Elsevier, Amsterdam
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalman G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Revision A.02 ed. Gaussian, Inc., Wallingford
Gao Y, Yang Y, Zheng W, Su Y, Zhang X, Roesky HW (2017). Inorg Chem 56:10220–10225
Lee C, Yang W, Parr RG (1988). Phys Rev B 37:785–789
Lewis GN (1916). J Am Chem Soc 38:762–785
Mulliken RS (1928). Phys Rev 32:186–222
Mulliken RS (1928). Phys Rev 32:761–772
Mulliken RS (1929). Phys Rev 33:730–747
Slater JC (1932). Phys Rev 41:255–257
Tian L, Feiwu C (2012). J Comput Chem 33:580–592
Van Vleck JH (1932). Phys Rev 41:208–215
Zubarev DY, BoIdyrev AI (2009). J Phys Chem A 113:866–868
Zubarev DY, Boldyrev AI (2008). J Organomet Chem 73:9251–9258
Funding
This work was supported by Applied Basic Research Project of Shanxi Province (Nos. 201801D221080, 201801D121257); Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2019L0814); Taiyuan Normal University Foundation for Leaders of Disciplines in Science; Shanxi “1331 project” Collaborative Innovation Center; Teaching reform project of Taiyuan Normal University (No. JGLX1815); and the Ph.D. Start-up Foundation of Taiyuan Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Huo, Rp., Zhang, X. & Zhang, Cf. A new look at the essence of the formation of trigonal planar coordination of the central N and O atoms. Struct Chem 31, 541–546 (2020). https://doi.org/10.1007/s11224-019-01423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01423-9