Abstract
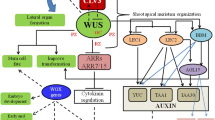
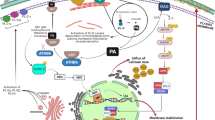
Peptidyl Prolyl Isomerases (PPIases) accelerate cis–trans isomerization of prolyl peptide bonds. In rice, the PPIase LRT2 is essential for lateral root initiation. LRT2 displays in vitro isomerization of a highly conserved W–P peptide bond (104W–P105) in the natural substrate OsIAA11. OsIAA11 is a transcription repressor that, in response to the plant hormone auxin, is targeted to ubiquitin-mediated proteasomal degradation via specific recognition of the cis isomer of its 104W–P105 peptide bond. OsIAA11 controls transcription of specific genes, including its own, that are required for lateral root development. This auxin-responsive negative feedback circuit governs patterning and development of lateral roots along the primary root. The ability to tune LRT2 activity via mutagenesis is crucial for understanding and modeling the role of this bimodal switch in the auxin circuit and lateral root development. We present characterization of the thermal stability and isomerization rates of several LRT2 mutants acting on the OsIAA11 substrate. The thermally stable mutants display activities lower than that of wild-type (WT) LRT2. These include binding diminished but catalytically active P125K, binding incompetent W128A, and binding capable but catalytically incompetent H133Q mutations. Additionally, LRT2 homologs hCypA from human, TaCypA from Triticum aestivum (wheat) and PPIB from E. coli were shown to have 110, 50 and 60% of WT LRT2 activity on the OsIAA11 substrate. These studies identify several thermally stable LRT2 mutants with altered activities that will be useful for establishing relationships between cis–trans isomerization, auxin circuit dynamics, and lateral root development in rice.








Similar content being viewed by others
References
Acevedo LA, Nicholson LK (2018) 1H, 13C and 15N NMR assignments of cyclophilin LRT2 (OsCYP2) from rice. Biomol NMR Assign 12:171–174
Acevedo LA, Kwon J, Nicholson LK (2019) Quantification of reaction cycle parameters for an essential molecular switch in an auxin-responsive transcription circuit in rice. Proc Natl Acad Sci USA 116:2589–2594
Anthis NJ, Clore GM (2013) Sequence-specific determination of protein and peptide concentrations by absorbance at 205 nm. Protein Sci 22:851–858
Boehr DD, Nussinov R, Wright PE (2009) The role of dynamic conformational ensembles in biomolecular recognition. Nat Chem Biol 5:789–796
Bosco DA, Eisenmesser EZ, Clarkson MW, Wolf-Watz M, Labeikovsky W, Millet O, Kern D (2010) Dissecting the microscopic steps of the cyclophilin a enzymatic cycle on the biological HIV-1 capsid substrate by NMR. J Mol Biol 403:723–738
Bossard MJ, Koser PL, Brandt M, Bergsma DJ, Levy MA (1991) A single Trp121 to Ala121 mutation in human cyclophilin alters cyclosporin a affinity and peptidyl-prolyl isomerase activity. Biochem Biophys Res Commun 176:1142–1148
Chrisman IM, Nemetchek MD, de Vera IMS, Shang J, Heidari Z, Long Y, Reyes-Caballero H, Galindo-Murillo R, Cheatham TE 3rd, Blayo AL, Shin Y, Fuhrmann J, Griffin PR, Kamenecka TM, Kojetin DJ, Hughes TS (2018) Defining a conformational ensemble that directs activation of PPARgamma. Nat Commun 9:1794
Compton LA, Davis J, Macdonald JR, BÄChinger HP (1992) Structural and functional characterization of Escherichia coli peptidyl-prolyl cis–trans isomerases. Eur J Biochem 206:927–934
Del Bianco M, Kepinski S (2018) Building a future with root architecture. J Exp Bot 69:5319–5323
Del Pozo JC, Manzano C (2014) Auxin and the ubiquitin pathway. Two players-one target: the cell cycle in action. J Exp Bot 65:2617–2632
Farrow NA, Zhang O, Forman-Kay JD, Kay LE (1994) A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J Biomol NMR 4:727–734
Fawzi NL, Ying J, Torchia DA, Clore GM (2012) Probing exchange kinetics and atomic resolution dynamics in high-molecular-weight complexes using dark-state exchange saturation transfer NMR spectroscopy. Nat Protoc 7:1523–1533
Fischer G (1994) Peptidyl-prolyl cis/trans isomerases and their effectors. Angew Chem 33:1415–1436
Freedberg DI, Ishima R, Jacob J, Wang YX, Kustanovich I, Louis JM, Torchia DA (2002) Rapid structural fluctuations of the free HIV protease flaps in solution: relationship to crystal structures and comparison with predictions of dynamics calculations. Protein Sci 11:221–232
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414:271–276
Greenwood AI, Rogals MJ, De S, Lu KP, Kovrigin EL, Nicholson LK (2011) Complete determination of the Pin1 catalytic domain thermodynamic cycle by NMR lineshape analysis. J Biomol NMR 51:21–34
Greenwood AI, Kwon J, Nicholson LK (2014) Isomerase-catalyzed binding of interleukin-1 receptor-associated kinase 1 to the EVH1 domain of vasodilator-stimulated phosphoprotein. Biochemistry 53:3593–3607
Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49:373–385
Hanes SD (2015) Prolyl isomerases in gene transcription. Biochim Biophys Acta 1850:2017–2034
Heckman KL, Pease LR (2007) Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc 2:924
Holliday MJ, Armstrong GS, Eisenmesser EZ (2015) Determination of the full catalytic cycle among multiple cyclophilin family members and limitations on the application of CPMG-RD in reversible catalytic systems. Biochemistry 54:5815–5827
Huynh K, Partch CL (2015) Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci 79:28.9.1–28.9.14
Ishikawa Y, Vranka JA, Boudko SP, Pokidysheva E, Mizuno K, Zientek K, Keene DR, Rashmir-Raven AM, Nagata K, Winand NJ, Bachinger HP (2012) Mutation in cyclophilin B that causes hyperelastosis cutis in American Quarter Horse does not affect peptidylprolyl cis-trans isomerase activity but shows altered cyclophilin B-protein interactions and affects collagen folding. J Biol Chem 287:22253–22265
Janowski B, Wöllner S, Schutkowski M, Fischer G (1997) A protease-free Assay for peptidyl prolylcis/transisomerases using standard peptide substrates. Anal Biochem 252:299–307
Jing H, Yang X, Zhang J, Liu X, Zheng H, Dong G, Nian J, Feng J, Xia B, Qian Q, Li J, Zuo J (2015) Peptidyl-prolyl isomerization targets rice Aux/IAAs for proteasomal degradation during auxin signalling. Nat Commun 6:7395
Kang B, Zhang Z, Wang L, Zheng L, Mao W, Li M, Wu Y, Wu P, Mo X (2013) OsCYP2, a chaperone involved in degradation of auxin-responsive proteins, plays crucial roles in rice lateral root initiation. Plant J 74:86–97
Kutschera U, Niklas KJ (2016) The evolution of the plant genome-to-morphology auxin circuit. Theory Biosci 135:175–186
Liu J, Chen CM, Walsh CT (1991) Human and Escherichia coli cyclophilins: sensitivity to inhibition by the immunosuppressant cyclosporin a correlates with a specific tryptophan residue. Biochemistry 30:2306–2310
Lu KP, Finn G, Lee TH, Nicholson LK (2007) Prolyl cis–trans isomerization as a molecular timer. Nat Chem Biol 3:619–629
Moreno-Risueno MA, Benfey PN (2011) Time-based patterning in development: the role of oscillating gene expression. Transcription 2:124–129
Motlagh HN, Wrabl JO, Li J, Hilser VJ (2014) The ensemble nature of allostery. Nature 508:331–339
Nechama M, Kwon J, Wei S, Kyi AT, Welner RS, Ben-Dov IZ, Arredouani MS, Asara JM, Chen CH, Tsai CY, Nelson KF, Kobayashi KS, Israel E, Zhou XZ, Nicholson LK, Lu KP (2018) The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat Commun 9:1603
Nicholson LK, Kay LE, Baldisseri DM, Arango J, Young PE, Bax A, Torchia DA (1992) Dynamics of methyl groups in proteins as studied by proton-detected 13C NMR spectroscopy. Application to the leucine residues of staphylococcal nuclease. Biochemistry 31:5253–5263
Nicholson LK, Yamazaki T, Torchia DA, Grzesiek S, Bax A, Stahl SJ, Kaufman JD, Wingfield PT, Lam PY, Jadhav PK et al (1995) Flexibility and function in HIV-1 protease. Nat Struct Biol 2:274–280
Normanly J (2010) Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb Perspect Biol 2:a001594
Pyott SM, Schwarze U, Christiansen HE, Pepin MG, Leistritz DF, Dineen R, Harris C, Burton BK, Angle B, Kim K, Sussman MD, Weis M, Eyre DR, Russell DW, McCarthy KJ, Steiner RD, Byers PH (2011) Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. Hum Mol Genet 20:1595–1609
Ramos JA, Zenser N, Leyser O, Callis J (2001) Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13:2349–2360
Sekhar A, Kay LE (2013) NMR paves the way for atomic level descriptions of sparsely populated, transiently formed biomolecular conformers. Proc Natl Acad Sci USA 110:12867–12874
Sekhon SS, Kaur H, Dutta T, Singh K, Kumari S, Kang S, Park SG, Park BC, Jeong DG, Pareek A, Woo EJ, Singh P, Yoon TS (2013) Structural and biochemical characterization of the cytosolic wheat cyclophilin TaCypA-1. Acta Crystallogr D 69:555–563
Sharaf NG, Gronenborn AM (2015) (19)F-modified proteins and (19)F-containing ligands as tools in solution NMR studies of protein interactions. Methods Enzymol 565:67–95
Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446:640–645
Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ (2001) AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13:2809–2822
Torchia DA (2011) Dynamics of biomolecules from picoseconds to seconds at atomic resolution. J Magn Reson 212:1–10
Torchia DA (2015) NMR studies of dynamic biomolecular conformational ensembles. Prog Nucl Magn Reson Spectrosc 84–85:14–32
Torchia DA, Batchelder LS, Fleming WW, Jelinski LW, Sarkar SK, Sullivan CE (1983) Mobility and function in elastin and collagen. Ciba Found Symp 93:98–115
Trivedi DK, Yadav S, Vaid N, Tuteja N (2012) Genome wide analysis of Cyclophilin gene family from rice and Arabidopsis and its comparison with yeast. Plant Signal Behav 7:1653–1666
Venditti V, Egner TK, Clore GM (2016) Hybrid Approaches to structural characterization of conformational ensembles of complex macromolecular systems combining NMR residual dipolar couplings and solution X-ray scattering. Chem Rev 116:6305–6322
Walsh CT, Zydowsky LD, McKeon FD (1992) Cyclosporin A, the cyclophilin class of peptidylprolyl isomerases, and blockade of T cell signal transduction. J Biol Chem 267:13115–13118
Waudby CA, Ramos A, Cabrita LD, Christodoulou J (2016) Two-dimensional NMR lineshape analysis. Sci Rep 6:24826
Zheng H, Li S, Ren B, Zhang J, Ichii M, Taketa S, Tao Y, Zuo J, Wang H (2013) LATERAL ROOTLESS2, a cyclophilin protein, regulates lateral root initiation and auxin signaling pathway in rice. Mol Plant 6:1719–1721
Zhu ZX, Liu Y, Liu SJ, Mao CZ, Wu YR, Wu P (2012) A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol Plant 5:154–161
Zydowsky LD, Etzkorn FA, Chang HY, Ferguson SB, Stolz LA, Ho SI, Walsh CT (1992) Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci 1:1092–1099
Acknowledgements
This work is dedicated in honor of Dr. Dennis Torchia and his pioneering contributions to the field of protein NMR spectroscopy and to the training of multiple generations of NMR spectroscopists. As one of those trainees, LKN is grateful for the lasting and significant impact of Dr. Torchia’s mentorship on her journey in science. This work was supported by the National Science Foundation Grant MCB-1615350 and by the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award (2T32GM008267) from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Acevedo, L.A., Korson, N.E., Williams, J.M. et al. Tuning a timing device that regulates lateral root development in rice. J Biomol NMR 73, 493–507 (2019). https://doi.org/10.1007/s10858-019-00258-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-019-00258-0