Abstract
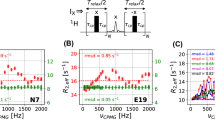
With the development of sophisticated pulsed field gradient- and phase cycling-approaches for suppressing certain coherence transfer pathways and selecting for others it is sometimes easy to forget that the process is not flawless. In some cases artifacts can emerge because unwanted transfers are immune to the phase cycle or the application of gradients. We consider here a simple 1H,13C HMQC pulse scheme and show that imperfections in the single 1H 180° refocusing pulse can give rise to small artifacts in methyl spectra that cannot be eliminated through extensive phase cycling or the use of gradients, but that are easily removed when the pulse is of the composite variety.


Similar content being viewed by others
References
Bax A (1985) A spatially selective composite 90o radiofrequency pulse. J Mag Reson 65:142–145
Bax A (1994) Multidimensional nuclear magnetic resonance methods for protein studies. Curr Opin Struct Biol 4:738–744
Bax A, Griffey RH, Hawkings BL (1983) Correlation of proton and nitrogen-15 chemical shifts by multiple quantum. NMR J Magn Reson 55:301–315
Bax A, Clore GM, Driscoll PC, Gronenborn AM, Ikura M, Kay LE (1990) Practical aspects of proton-carbon-carbon-proton three-dimensional correlation spectroscopy of 13C-labeled proteins. J Magn Reson 87:620–627
Bodenhausen G, Freeman R, Turner DL (1977) Suppression of artifacts in two-dimensional J spectroscopy. J Magn Reson 27:511–514
Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ (1996) Protein nmr spectroscopy: principles and practice. Academic Press, San Diego
Ernst RR, Bodenhausen G, Wokaun A (1987) Principles of nuclear magnetic resonance in one and two dimensions. Oxford University Press, Oxford
Freeman R, Keller J (1981) Suppression of artifacts in two-dimensional J Spectra. J Magn Reson 43:484–487
Freeman R, Kempsell SP, Levitt MH (1980) Radiofrequency pulse sequences which compensate their own imperfections. J Magn Reson 38:453–479
Huang R, Perez F, Kay LE (2017) Probing the cooperativity of Thermoplasma acidophilum proteasome core particle gating by NMR spectroscopy. Proc Natl Acad Sci USA 114:E9846–E9854. https://doi.org/10.1073/pnas.1712297114
Kay LE, Bax A (1990) New methods for the measurement of NH-CaH coupling constants in 15N-labeled proteins. J Magn Reson 86:110–126
Kay LE, Prestegard JH (1987) Methyl group dynamics from relaxation of double quantum filtered NMR signals-application to deoxycholate. J Am Chem Soc 109:3829–3835
Kay LE, Torchia DA, Bax A (1989) Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry 28:8972–8979
Kay LE, Bull TE, Nicholson LK, Griesinger C, Schwalbe H, Bax A, Torchia DA (1992) The measurement of heteronuclear transverse relaxation times in AX3 spin systems via polarization transfer techniques. J Magn Reson 100:538–558
Levitt M, Freeman R (1978) NMR population inversion using a composite pulse. J Magn Reson 33:473–476
Montelione GT, Wagner G (1990) Conformation-independent sequential NMR connections in isotope-enriched peptides by H-1C-13-N15 triple resonance experiments. J Magn Reson 87:183–188
Mueller L (1979) Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J Am Chem Soc 101:4481–4484
Religa TL, Sprangers R, Kay LE (2010) Dynamic regulation of archaeal proteasome gate opening as studied by. TROSY NMR Science 328:98–102. https://doi.org/10.1126/science.1184991
Rosenzweig R, Kay LE (2014) Bringing dynamic molecular machines into focus by methyl-TROSY NMR. Annu Rev Biochem 83:291–315
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34:93–158
Tugarinov V, Hwang P, Ollerenshaw J, Kay LE (2003) Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc 125:10420–10428
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. LEK holds a Canada Research Chair in Biochemistry. The author is grateful to Dr. Rui Huang for preparation of figures and to both Drs. Huang and Tairan Yuwen for useful discussions. LEK acknowledges a reviewer who pointed out that Freeman and Keeler had observed similar artifacts in J-resolved spectra 40 years ago.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kay, L.E. Artifacts can emerge in spectra recorded with even the simplest of pulse schemes: an HMQC case study. J Biomol NMR 73, 423–427 (2019). https://doi.org/10.1007/s10858-019-00227-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-019-00227-7