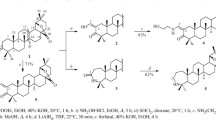
Sequential transformations of dipterocarpol (oximation, first-order Beckmann rearrangement, dehydration, isomerization) synthesized the new dammarane-type triterpenoid A-azepanodammara-20(21),24(25)-diene. Its cytotoxicity was studied in vitro against 60 cell lines of 9 human tumor types. The cytotoxicity against all cancer cell types increased significantly as compared to native dipterocarpol if an azepane fragment was introduced into ring A (percent inhibition from 15.72% to 82.08%). Detailed cytotoxicity studies confirmed the activity against a broad spectrum of human tumor cell lines with a mean (MID) log LC50 of 5.57.
Similar content being viewed by others
References
H. Zhang, P. Zhu, J. Liu, X. Yang, S. Xu, H. Yao, J. Jiang, W. Ye, X. Wu, and J. Xu, Eur. J. Med. Chem., 87, 159 (2014).
R. Mukherjee, M. Jaggi, P. Rajendran, M. J. Siddiqui, S. K. Srivastava, A. Vardhan, and A. C. Burman, Bioorg. Med. Chem. Lett., 14, 2181 (2004).
R. W. Zhang, W. Wang, H. Wang, and Y. Zhao, “Novel Ginsenoside Compounds, Compositions, and Methods of Use,” WO076900A1 (2008).
G. Chen, W. Song, X. Yang, H. Ge, and J. Li, “Protopanaxadiol Derivative and Application Thereof,” CN103387596A (2013).
G.-F. Zha, K. P. Rakesh, H. M. Manukumar, C. S. Shantharam, and S. E. Long, J. Med. Chem., 162, 465 (2019).
N. I. Medvedeva, O. B. Kazakova, T. V. Lopatina, I. E. Smirnova, G. V. Giniyatullina, I. P. Baikova, and V. E. Kataev, J. Med. Chem., 143, 464 (2018).
O. B. Kazakova, G. V. Giniyatullina, N. I. Medvedeva, T. V. Lopatina, I. P. Baikova, G. A. Tolstikov, and G. N. Apryshko, Bioorg. Khim., 40, 217 (2014).
E. F. Khusnutdinova, I. E. Smirnova, G. V. Giniyatullina, N. I. Medvedeva, E. Y. Yamansarov, D. V. Kazakov, O. B. Kazakova, P. T. Linh, Q. Viet, and D. T. Huong, Nat. Prod. Commun., 11, 33 (2016).
M. Ukiya, T. Kikuchi, H. Tokuda, K. Tabata, Y. Kimura, T. Arai, Y. Ezaki, O. Oseto, T. Suzuki, and T. Akihisha, Chem. Biodiversity, 7, 1871 (2017).
T. Akihisia, H. Tokuda, M. Ukiya, T. Suzuki, Enjo, K. Koike, T. Nikado, and H. Nishino, Chem. Pharm. Bull., 52, 153 (2004).
K. Hjrabayashi, S. Iwata, and H. Matsumoto, Chem. Pharm. Bull., 39, 112 (1991).
F. Inada, M. Somekawa, and H. Murata, Chem. Pharm. Bull., 41, 617 (1993).
G. V. Platonov, A. D. Zorina, M. A. Gordon, N. P. Chizhov, L. V. Balykina, Yu. D. Mikhailov, D. R. Ivanen, Tran Kim Kvi, and A. G. Shavva, Khim.-farm. Zh., 2, 42 (1995).
L. Revesz, P. Hiestand, L. La Vecchia, R. Naef, H.-U. Naegeli, L. Oberer, and H.-J. Roth, Bioorg. Med. Chem. Lett., 9, 1521 (1999).
Do Thi Thu Huong, Tran Thi Thu Thuy, Tran Thi Hien, Nguyen Thanh Tra, Nguyen Quyet Tien, I. E. Smirnova, O. B. Kazakova, E. M. Minnibaeva, and G. A. Tolstikov, Chem. Nat. Compd., 49, 58 (2013).
I. E. Smirnova, O. B. Kazakova, D. T. T. Huong, E. M. Minnibaeva, A. N. Lobov, and K. Yu. Suponitsky, Nat. Prod. Commun., 9, 1417 (2014).
M. C. Alley, D. A. Scudiero, P. A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker, and M. R. Boyd, Cancer Res., 48 (3), 589 (1988).
M. R. Grever, S. A. Schepartz, and B. A. Chabner, Semin. Oncol., 19, 622 (1992).
M. R. Boyd and K. D. Paull, Drug Dev., 34, 91 (1995).
R. H. Shoemaker, Nat. Rev. Cancer, 6, 813 (2006).
L. Q. Tran and K. Q. Tran, J. Chem., 36, 8 (1998).
Acknowledgment
The work was performed on State Task Topic No. AAAA-A17-117011910023-2. We thank the National Cancer Institute for determining the in vitro antitumor activity of 1 and 3–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2019, pp. 760–765.
Rights and permissions
About this article
Cite this article
Smirnova, I.E., Petrova, A.V. & Kazakova, O.B. Synthesis and Cytotoxicity of A-Azepanodammaradiene. Chem Nat Compd 55, 883–889 (2019). https://doi.org/10.1007/s10600-019-02838-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02838-w