Abstract
Catalysts used for selective catalytic reduction of NOx on diesel engines were deactivated for 890 and 2299 h in the exhausts from a diesel engine running on Swedish diesel fuel mark 1. The deactivated catalytic monoliths (100 mm long) were cut in 10 mm pieces along their axis and were characterized by physicochemical methods as well as by determination of the activity in the reduction of NOx by NH3 and in the oxidation of NH3 by O2.
Graphic Abstract

Similar content being viewed by others
1 Introduction
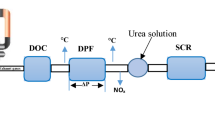
The selective catalytic reduction (SCR) technique, for reduction of NOx with ammonia is today a compulsory method for mobile applications like marine and automotive diesel engines. Marine engines run under conditions, which resemble the ones in stationary diesel power plants. In 2017, there are hundreds of SCR units installed on ships [1].
We contributed to the early development of the SCR technology for use on trucks using urea as reducing agent [2]. In 1996 a publication of the evaluation of the 1st SCR plants in Sweden, some of which were based on diesel engines, was published [3]. Kamata et al. [4] showed the effect of phosphorus on the acidity of the catalyst and on its activity since phosphorus is a common poison in diesel applications. We later studied the thermal stability of vanadia-based SCR catalysts for the use in diesel applications [5] since these catalysts could experience high temperature sintering during use.
The lubricating oil for the diesel engine is often a source for deactivation components for the catalyst. A SAE 15W-40 oil contained S 0.26, Ca 0.25, Zn 0.068 and P 0.061 wt% [6]. Nicosia et al. [7] studied the deactivation by calcium, phosphate and potassium on V2O5/WO3–TiO2 catalysts for diesel systems. They showed that Ca ions (introduced as calcium acetate), at 0.4 mol% based on vanadium, decreased the conversion of NO from 98 to 65% at 400 °C. When SO42− was present (introduced by feeding SO2 at 400 °C for 5 h), the deactivation was almost unnoticeable. Kröcher and Elsener [8] studied more carefully the effect of lubricating oil components (Ca, Mg, Zn, P, B and Mo), impurities in diesel fuel (K from rapeseed methyl ester) and impurities in the urea solution (Ca and K) on the activity of vanadia based SCR catalysts. The catalysts were impregnated with single components (i.e. Ca as acetate). Similar effect as presented by Nicosia [7] was obtained for Ca. Nicosia et al. [9] characterized the deactivated catalysts by NH3-TPD and found that the fresh catalyst desorbed 3.4 mg/g NH3 while Ca-poisoned one desorbed 2.0 mg/g. Thus, a decrease in the number of acid sites was proven. By using diffuse reflectance infrared fourier transform spectroscopy (DRIFTS) of adsorbed ammonia, it was possible to distinguish between Lewis and Brønsted acid sites. It was only the Brønsted sites that were affected by poisoning.
The influence of phosphorous, alkaline and alkaline earth metals as well as Cu and Cr on SCR was studied, in a high-throughput study, by Klimczak et al. [10]. Effects of impurities in biodiesel (K, Na, P), in urea solution (K, Na, Ca, Mg) and components from abrasion of the engine (Cr, Cu) on catalyst activity were reported. Different methods for catalyst doping, i.e. impregnation and poisoning by aerosols were used. The results from impregnation with nitrates show that at a loading of 0.5 mmol/g washcoat the activity was decreased by 75% for Ca at 350 °C. When an aerosol from Ca(NO3)2 was introduced at 500 °C and the activity measured at 350 °C, a decrease in conversion by 23% was observed. The amount of adsorbed NH3 was 1.9 ml/g for the fresh catalyst and 1.5 ml/g for the one containing Ca, i.e. a decrease in ammonia adsorption capacity by 21%. The effect of deposition by aerosols is thus much smaller than that using impregnation. Maunula et al. [11] studied SCR catalysts in mobile.
off-road applications. They found that the aged catalysts after 3 000 and 8 000 h had axial accumulation of the elements (P, Zn, Ca, Na, K, S, Si and Fe) coming from lubrication oil and fuel oil. Only the front part of the catalyst had higher concentrations of poisons, which correlates to the decreased conversion of NOx. Pietkäinen et al. [12] compares the contents of poisons in rapeseed oil (RSO) to that in diesel fuel oil and shows especially high amounts of P and Ca. After running the engine on RSO for 61 h at 340 °C the reduction of NOx was reduced by 60%. Ca and P probably primarily caused this effect.
Schobing et al. [13] studied the deactivation by Ca, Zn and P of a commercial 2% vanadia and 3% tungsta on TiO2 full body catalyst. They found the when 0.24 wt% Zn was added the activity at 225 °C decreased by 18%. For Ca the effect was 74%. Above 300 °C no deactivation was observed. P poisoning at the 1 wt% level with P decreased the maximum conversion of NOx from 98 to 85%. Castellino et al. [14] show that phosphorous added to the exhaust gas in for of aerosols deactivate SCR catalyst more than when wet impregnated. Our materials consist of thin layers of catalytic active components supported on cordierite so the effect of deactivation will be easier detected in our experiments.
It is also important that catalysts used in diesel applications do not produce large amounts of N2O. Cho et al. [15] compare NOx reduction and N2O emission in diesel exhausts using Fe-zeolite and vanadium based catalysts. They found that when zeolite catalyst was used the N2O/NOx ratio was its maximum at almost 16% at 300 °C. When V2O5–WO3/TiO2 was used 2% was the maximum value at 200 °C decreasing at higher temperatures. Since N2O is a very strong green house gas we have studied its formation here. At high temperatures ammonia is directly oxidised to N2O and NO which was already found in the 1990:ies [16]. Efstathiou and Fliatoura [16] used an 8% V2O5/TiO2 catalyst and determined the N2 formation rates in The SCR as well as in the NH3 oxidation reactions. At 300 °C, 700 μmol/min/g of N2 was formed in the SCR reaction but only 17 μmol/min/g of N2 in the oxidation of NH3 to N2. Duffy et al. [17], using isotopic methods, studied the effect of water on the formation of products in the SCR and the ammonia oxidation reactions. So did also Ozkan et al. [18] using TPD technique to determine the reaction products.
There is not very much detailed information in literature on the changes of pore structure and catalyst activity when the catalyst is used in real diesel engine exhausts. Therefore, there is still need for more information on these effects. The aim of this study is to scan the effect on activity of vanadia on titania catalysts used for 890 and 2299 h and compare it to fresh catalysts. We intend to show how the deactivation process effects pore structure and activity and to correlate the content of poisons to its activity and selectivity. We will also show how the poisons distribute along the monolith axis at various times of use.
2 Methods
2.1 Preparation of the Catalyst
We used a cordierite monolithic support, 5.66 × 6″, with 400 channels per square inch and a wall thickness of 6 mils from Corning Glassworks. Two cores, each 100 mm long with a diameter of 25 mm, were drilled from the monolith and used in the deactivation test. The rest of the monolith was used as a reference for determination of the activity of the fresh catalyst.
The amount of active phase used was 5.64% V2O5 on TiO2 [Rhone Polenc DT(8)] corresponding to 60% of a monolayer. After impregnating the titania with ammonium meta vanadate in aqueous oxalic acid the catalyst was dried for 15 h at 125 °C. Calcination was then performed at 450 °C for 1.25 h in a flow of air. The calcined material was ground to fine particles and suspended in water to form a slurry. The monoliths were dipped in the active material slurry removing the surplus by air blowing. Drying was performed at 110 °C for 1 h. The process was repeated until 18.2 wt% of the titania based vanadia was reached.
2.2 Deactivation Procedure
The deactivation was performed in a diesel engine rig. The fuel was Swedish class 1 diesel. The engine rig was equipped with a 10 cm long monolith dummy placed in the exhaust gas stream. The samples for deactivation were placed in drilled, 2.5 cm diameter, holes in the dummy. The deactivation cycle was 1 h long and the mean temperature was 470 °C with large variation in temperatures, which should represent 2 years of mixed driving of the vehicle. The maximal temperature reached was 570 °C for about 7.5 min in each cycle. The accelerated test was supposed to simulate the normal running of a truck for a distance of up to 500 000 km.
2.3 Measurement of Catalyst Activities
Before measurement of the activity the centre part (9 × 9 channels wide) of the 10 cm long and 2.5 cm wide deactivated catalysts was taken for catalytic activity one channel close to it was taken for analysis by XPS. The centre part was cut into 1 cm long pieces along the axis. Activity measurements in the SCR reaction were performed in a micro reactor made of quartz glass. One piece of the catalyst, 9 × 9 channels wide (~ 1 × 1 cm) and 1 cm long, weighing about 1.0 g, was mounted in the reactor. Quartz wool was used to support the monolith and to hinder the reaction gas to bypass the sample. The gas composition for the SCR reaction was 200 ppm NO, 200 ppm NH3, 3.3% O2 with a balance of nitrogen. The gas flow was 3 000 Ncm3/min. The space velocity thus obtained was about 180 000 h−1. The gases were taken from cylinders of known composition and were blended in an Environics series 2000 computerised multi-component gas mixer containing 8 mass flow regulators.
The total pressure in the system was 1.45 bar. A testing scheme consisted of measurements at 150–500 °C at 50 °C intervals. The inlet concentration was measured for 15 min and the outlet concentration for 45 min at each temperature. The procedure was controlled by a PC where the data were also gathered at 10 s interval. The analysis of NOx was made by chemiluminescence using an ECO Physics 700 CLD EL ht instrument. The analysis of N2O was made by NDIR with an Ultramat 5 E from Siemens. A thermocouple measured the temperature of the catalyst block.
The oxidation of NH3 was studied in separate experiments using a mass spectrometer with He as background gas in order to determine the oxidation products with an equipment as described before [19]. Piece 5 of the catalysts was used at 700 ppm NH3, 2% O2 and a pressure of 1.24 bar (space velocity 45 000 h−1). The temperatures used were 340–460 °C with an interval of 20 °C.
The activity in the SCR reaction is presented as an apparent rate constant determined from the following equations:
where:
CNOin = concentration of NO in inlet to reactor (mol/cm3)
CNOout = concentration of NO in outlet from reactor (mol/cm3)
where:
kNO = first order rate constant (1/s), based on catalyst weight
FNOin = molar flow of NO into the reactor (mol/s)
kNO0 = pre-exponential factor (cm3/s/gcat)
Ea = activation energy (J/mol)
R = universal gas constant (J/mol/K)
T = temperature (K)
mcat = mass of catalyst (g)
For the NH3 oxidation experiments
CNH3in = concentration of NH3 in inlet to reactor (mol/cm3)
CNH3out = concentration of NH3 in outlet from reactor (mol/cm3)
where
The kinetics for the oxidation reactions of NH3 are given by:
The apparent rate constants and activation energies are presented in the result section.
2.4 Measurements of the Composition by XPS
The centre part (9 x 9 channels wide) of the 10 cm long deactivated catalysts was analysed by XPS. One channel was cut open and the surface of the inside of the channel was analysed by a Kratos XSAM 800 equipped with a Mg anode (1253.6 eV). Quantitative analysis was performed by the program DS 800 data system. Kratos supplied the sensitivity factors. The analysis was performed along the axis of the monolith on almost 20 points. The majority of test points were concentrated to the inlet portion of the monolith. The same analysis was performed on the cordierite monoliths used from 890 to 2299 h. No analysis of the shift in peak values in the spectra was performed.
2.5 Surface Area and Pore Structure by N2 Adsorption
The surface area and the pore size distribution of fresh and deactivated catalysts as well as the cordierite were determined by physisorption of N2 at − 196 °C using a Micromeritics ASAP 2400 system. The study of the pore system was performed on a piece of the wall of each of the 1 cm monoliths, used for activity measurements. Outgassing was performed at 0.04 mm Hg and 90 °C for 1 h and at 400 °C for 24 h to a final pressure of 10−3 Torr before the adsorption study. The BET surface area was calculated as described by Brunauer, Emmett and Teller [20]. The accuracy of this method was better than 1%, according to separate measurements for the experimental conditions used. The mesopore size distribution was calculated using the method described by Barettet al. [21]. The total pore volume is given for pores smaller than 3000 Å in diameter.
The micropore volume and the micropore surface area were calculated using the t-plot method of Harkins and Jura [22].
3 Results and Discussion
3.1 Surface Area and Pore Structure
3.1.1 Surface Area of the Cordierite
The BET surface area of the cordierite is 0.489 m2/g. The area of pores below 17 Å is only 0.073 m2/g. The total pore volume (by N2) is 0.00081 cm3/g (d < 1840 Å). The micropore volume (d < 17 Å) is only 0.000016 cm3/g or 2%. We note that 20% of the pore volume is in pores with d > 1280 Å, 27% between 463 and 1280 Å and 41% below 463 Å. There are probably also large macropores (d ≫ 500 Å) but we have not measured them by Hg porosimetry here.
The cordierite is manufactured in a way to yield a compact material with high strength and a wide distribution of pores (not shown here). Considering the low pore volume only very small amounts of the active phase is supposed to be deposited inside the pores of the cordierite. There will be an active catalyst layer build up on the surface of the cordierite channels. The thickness of this layer is estimated to around 20 μm.
3.1.2 Surface Area of the Fresh and Used Catalysts
The BET surface area (SBET) for the fresh material (including the cordierite support) varies somewhat with position in the monolith. The 1st piece has a surface area of 8.55, the 2nd 12.65 while the rest (pieces 3–10) of the fresh monolith has a surface area of around 13.06 ± 0.53 m2/g. The surface areas measured include the surface area of the cordierite material, which is only about 0.49 m2/g. Since the amount of active material is 18.2% then the calculated surface area of the active material is 69.6 m2/g. This is in line with other results for rather high concentration vanadia titania catalysts [23].
Table 1 shows that there is a decrease of SBET by 41.8% after 890 h and 47.2% after 2299 h for the majority of the pieces. Thus, the surface area is changed considerably during the test procedure. This could be an effect of sintering during the high temperature parts of the deactivation cycle or from deposition of poisons. There is a definite increase in surface area from the 1st piece towards the end of the monolith. The average surface areas are 13.06 ± 0.53, 7.59 ± 0.22 and 6.89 ± 0.25 m2/g for the fresh, 890 h and 2299 h deactivated materials from the 3rd piece on. There is a general trend of decreasing surface area with increasing deactivation time as shown in Table 1. The low value of the surface area for the 1st piece of the fresh catalyst could be due to a smaller amount of active material on this piece caused by an error in the preparation method. An investigation by Liu et al. [24] on a field returned catalyst used for 256 000 km on a diesel truck showed a decrease in surface are by 15%. This catalyst was protected from temperatures above 500 °C which could explain the low change compared to ours.
The value of the surface area for the 1st piece is only 65% of the value for the mean of pieces 3–10 of the fresh catalyst. There is no reason why the surface area should vary along a fresh catalyst. Therefore, we assume that this piece contains 35% less active material on the cordierite caused by the way of preparation. This catalyst is a supported one and not a full monolith.
There is always a much larger decrease in surface area after deactivation of the 1st piece than for the others. For the 1st piece the decrease is 73% after 890 h and only 38% for the 6th piece. The surface area stabilizes at around the 4th piece for the deactivated catalysts (7.60 ± 0.22 at 890 h and 6.89 ± 0.26 m2/g at 2299 h). The decrease of the surface area for the 1st piece is large during the 1st 890 h (− 71.4%) with only a minor additional decrease up to 2299 h (− 76.6%). This indicates that thermal sintering proceeds much slower after 890 h which is in line with earlier studies [5]. The two 1st pieces of the sample used for 2299 h has higher surface areas than the ones used for only 890 h. The results of Castellino et al. [14] who introduced aerosols of P4O10 show that the total surface area decreased by 47% in the 5 1st cm of the monolith while only with 17% in the last 5 cm using a 100 ppm H3PO4 water solution for generation of the aerosol. Comparable values for our study were 72% for the 1st 1 cm and 42% from 3 to 10 cm.
Madia et al. [23] have studied the thermal stability of vanadia-tungsta-titania catalyst with vanadia contents from 0 to 3%. When aging the 3% catalyst for 100 h at 550 °C and 30 h at 600 °C the surface area dropped from 72 to 33 m2/g (− 54%). Our recalculated values from Table 1 gives 71.7, 41.7 and 37.9 m2/g for the fresh, used for 890 and 2299 h respectively. The decrease after 890 h is 41.8% (estimated time in the deactivation rig at 570 °C is 111 h) and after 2299 h it is 47.1%. Their values compare to ours especially since the higher vanadia content of our catalyst should lead to a higher degree of sintering [5].
3.1.3 Pore Structure of the Catalysts
Table 2 shows that, the total pore volume of the fresh catalyst increases by 34% from the inlet towards the outlet of the monolith. The value for the active phase (V2O5/TiO2) only was 0.3198 cm3/g (fresh catalyst 9th piece). The micropore volume is low for all samples. The average pore diameter is lowest for the fresh catalyst increasing by 38–57% with use for different pieces. The micropore surface area increases along the axis showing that some compounds are accumulating in the small pores in the front of the catalyst monolith. It is not possible to evaluate how the micropore system is affected by time of use because of lower accuracy of these data.
The pore size distribution of the 1st piece of the fresh catalyst has a bimodal structure with two maxima at 140 and 320 Å diameter (Fig. 1). The rest of the monolith has only a single maximum around 130 Å. Thus, it is clear that the pore system is more open one in the 1st piece. This would lead to an increased rate of diffusion and could affect the activity positively if there were any pore diffusion limitations in the catalyst layer.
Figure 2 shows that the 1st piece of the fresh monolith has a smaller amount of the surface area than piece 4 in pore smaller than 150 Å. This supports our conclusion that the poison penetrates into the smaller meso-pores and at least partially into the micro-pores of the catalyst layer. Comparison of the cumulative surface area in Fig. 2 to the BET surface area in Table 1 shows that most of the area is in meso-pores for piece 1 of the fresh catalyst. This can also be observed in Table 2 where micro-pore surface areas (dpore < 17 Å) are low. This is also true for piece 4 which is representative for the rest of the monolith.
Figure 3 shows the effect of time of use on the pore size distribution. For the 1st piece there is a bimodal pore distribution with maxima at 138 Å and 303 Å for the fresh material. On use the peak at 138 Å disappears and a maximum is obtained at larger pore sizes. For piece 4, which is representative for most of the catalyst pieces, the fresh material has the same peak at 138 Å but with a 1.9 × higher value than for the 1st piece. Thus, it is a more open pore system. The trend of increasing maxima in pore size on use is seen on piece 4 as on piece 1.
3.2 Chemical Composition of the Surface of the Catalyst Materials
3.2.1 Cordierite
There is a somewhat higher accumulation of diesel soot in the 1st 25 mm from the inlet of the monolith as shown by the C/Si ratio in Fig. 4. The soot penetrates the whole length though. In addition, an accumulation of Ca is observed since the ratio Ca/Ti (2nd axis) is 0.23 at 1 mm and decreases to 0.08 at 95 mm (piece 10). The O/Si ratio is 3.47 ± 0.17 as for synthetic cordierite [25].
The concentrations of the poisons (max P/Si = 0.28) in the inlet of the monolith are P > Ca > Zn > S (Fig. 5). Al is present in a ratio to Si which is about half of the one expected from the composition of the cordierite (0.22). P/Si and Ca/Si seem to follow each other and decrease with position along the axis of the monolith, more slowly beyond 20 mm. The is probably some kind of calcium phosphate formed on the catalyst. Possible compounds at 300 °C are: CaH2P2O7, Ca3(HP2O7)2 and Ca2HP3O10 [26]. Zn/Si has a sharp decline in concentration for the 1st piece from 0.11 in the front to a mean value (from 20 mm on) of 0.055 with a standard deviation of 0.0062 for the rest of the monolith. S is present at a low (0.02–0.03 S/Si ratio) and a mean value of 0.022 with a standard deviation of 0.0047 from 20 to 95 mm when the outlier at 35 mm is removed. One could expect an amount of CaSO4 on the surface at a Ca/Si ratio of 0.022. The rest (0.105 Ca/Si ratio) should form some compound with P.
3.2.2 Vanadia on Titania Catalyst
Soot covers the catalyst to a similar amount for 890 and 2299 h (Fig. 6). The amount in the inlet is about 8 × larger than from 25 mm onwards. Diesel soot penetrates the whole length of the monolith.
There is an increased concentration of catalyst poisons in the 1st 6 mm of the catalyst front (Fig. 7). The values are P/Ti = 5.5, Ca/Ti = 7.5, Zn/Ti = 1.6 and S/Ti = 0.9 at 890 h at 1 mm. At 2299 h they are P/Ti = 5.7, Ca/Ti = 3.0, Zn/Ti = 3.1 and S/Ti = 3.5. Further, into the monolith there is a decrease in concentration of poisons. After the 1st 6 mm, values tend to stabilize at constant values. Liu et al. [24] observed high concentration of P in the inlet section of the catalyst dropping sharply down the monolith. Zn on the other hand remained almost constant through the monolith while ours results show a clear accumulation in the catalyst front (Fig. 7).
In the inlet, the P/Ti is about the same at 890 and 2299 h. From 7 mm onwards, the P/Ti ratio is about 1 for 890 h and 0.5 for 2299 h. Ca also behaves in a strange way being highest for 890 h up till 6 mm but increasing with time of use, as expected, further down the monolith. Both the S/Ti and Zn/Ti are lowest at 890 h increasing more or less in proportion to time of use. The data for concentrations of the part of the monolith between 20 and 100 mm show that the ratio P/Ti is always highest (around 0.8) followed by Ca/Ti (around 0.5) and Zn/Ti and S/Ti at around 0.13 (not shown here).
When comparing the cordierite monolith with the vanadia-titania one it is clear that the penetration of poisonous compounds along its axis is larger for the cordierite. Gradients for calcium are obtained up to 40 mm from the inlet in that case.
Castellino et al. [14] have studied commercial V2O5–WO3/TiO2 SCR monoliths showing results after 453 and 1000 h of aerosol deposition. After 453 h, the amount of P was 0.7 wt% at 5 mm and 0.5 wt% at 45 mm from the inlet. Another sample showed 1.8 wt% at 10 mm and 1.3 wt% at 40 mm after 1000 h. Their results resemble ours in Fig. 7. The accumulation of S and Ca was very small and could only be detected in the inlet of the monolith. This also resembles our results.
3.3 Catalyst Activity in the SCR of NO with NH3
As seen in Table 3 the decrease in activity, at 350 °C, is very large in the 1st piece going down to only 3% of the fresh ones after 2299 h. The loss of activity presented by Castellino et al. [14] in the experiments mentioned above is 2.5% by phosphorous alone. From piece 3 on the decrease in activity is almost independent on the position in the monolith. About 50 and 25% of the original activity is retained after 890 and 2299 h.
At 350 °C the rate constant for the 1st piece of the fresh catalyst is 24% larger than the mean of pieces 3–10. The SBET is at the same time 35% lower than the mean of the rest of the monolith. Thus, an explanation of lower amount of active material in piece 1 is plausible.
When the normalized reaction rate is plotted as a function of the time of use (not shown here) a curve typical for a system where poisoning effects prevails and not for a system where pore plugging is dominating [27].
As can be seen in Table 4 there is a maximum in the rate constant at around 350 °C for the fresh catalyst. Negative rate constants are obtained at high temperatures meaning that the oxidation of ammonia is observed. As seen in the table a more deactivated catalyst has a smaller tendency to lose activity at high temperatures.
Kamata [4] impregnated a commercial catalyst with the composition V:W: Ti = 0.012:0.028:0.96, with aqueous solutions of H3PO4 to yield materials with 0–5% P2O5. Their values of P/Ti by XPS were in the range 0–0.0375 obtained by impregnation with p containing compounds. Ours are much larger (0–2.5) since we have a preferential surface deposition of P. At 0.5 wt% P their relative rate constant was 0.76 at 1 wt% P. The values of the rate constants were 673 for the fresh and 210 cm3/g/s for the material with 1% P at 350 °C. Their rate constants were about 10 × higher for the fresh catalyst, which can be explained by the small particle size used in Kamata’s experiments (100–180 μm).
There is an early study [28] of the effect of 3% Zn at 180 °C. A fresh catalyst had a conversion of NOx of 84.5% whereas the Zn poisoned one had a conversion of 76%. This compound has only a moderate effect on activity.
In order to separate effects of sintering from the effects of poisoning surface specific rate constants were evaluated and are shown in Fig. 8. The 1st piece for the fresh catalyst has a constant of 5.4 while the mean is 1.67 ± 0.15 cm3/m2/s for piece 4–10. The catalyst used for 890 h has a higher rate constant per surface area than the other ones except at the exit of the monolith. The values for the fresh and the one used for 2299 h are very close to each other meaning that the change in surface area is a major factor in the change in activity.
The surface specific rate constant varies with temperature and with position in a specific manner (Fig. 8). At temperatures below 350 °C we observe an increased rate constant for the 890-h used material compared to the fresh catalyst. This is in line with our earlier studies on the positive effect of sulphating of the catalyst surface [29, 30]. Above 400 °C the activity of the catalyst falls with the time of use or the degree of deactivation as expected.
Figure 9 shows how the surface specific rate constant for the fresh catalyst shifts its maximum from 350 °C for the 1st piece to 450 °C for the rest of the pieces. The experimental value of the rate constant is highest for the 1st piece (2.38 cm3/m2/s) and decreases along the monolith. As seen above piece 1 probably contains only 65% of the amount of active material compared to the other ones. When corrected for this the new value is 1.55 cm3/m2/s, which is within 10% of the values for the other pieces. When used for 890 h the maximum (0.367 cm3/m2/s) is at 350 °C for the most poisoned catalyst (piece 1), and is highest (0.70 cm3/m2/s) at 450 °C for the least poisoned material (piece 10). After 2299 h the values of maximal rate constants are: 0.147 cm3/m2/s for piece 1 at 400 °C, 0.473 cm3/m2/s for piece 2 at 400 °C, 0.570 cm3/m2/s for piece 5 at 400 °C and 0.705 cm3/m2/s for piece 10 at 450 °C. Thus, the decrease in activity at this time of use follows a trend with lowest activity in the inlet of the catalyst as would be expected from the penetration of poisons.
Another trend is also obvious; there is an increased activity of the poisoned samples below 300–350 °C compared to the fresh catalyst for both 890 and 2299 h. The trend is particularly clear for 890 h. Thus, an activation occurs after some time on stream as described above.
Chen et al. [31] showed that P and Ca deactivated a 5% vanadia/TiO2 by 23% at Ca or P/V = 0.3 and 300 °C. Their rate constants were 10.5 for the fresh and 8.1 cm3/g/s for the deactivated catalyst. Ours were 5 × larger.
The apparent activation energy for the 1st piece was 49.8 for the fresh catalyst and 46.8 kJ/mol for the one used for 2299 h between 150 and 250 °C. The 5th piece´s values were 53.6, 44.6 and 45.7 kJ/mol for 0, 890 and 2299 h of use. Thus, the more poisoned material (1st piece used 2299 h) has the lowest activation energy as shown before [19]. The 9th piece´s values were 57.7, 73.3 and 64.4 kJ/mol for 0, 890 and 2299 h of use. Amiridis and Solar [31] have found that Eaapp is dependent on the amount of vanadia in the catalyst. At 1% V2O5 Eaapp was 65 kJ/mol and at 6.6% V2O5 it was 40 kJ/mol. These data support the ones presented here for our 5.64% catalyst (49.8 kJ/mol). The pre-exponential factors for the 1st piece are: 8.25 × 104 and 3.48 × 103 cm3/m2/s after 0 and 2299 h of use respectively. Thus, after 2299 h only 4% of the activity remains. This compares well with the values in Table 3. The pre-exponential factors for the 9th piece is 1.13 × 105, 5.83 × 106 and 6.48 × 105 cm3/m2/s after 0, 890 and 2299 h of use respectively. Here the activation by poisoning by sulphate groups is clearly seen increasing activity after 890 h and even after 2299 h of use.
A clear tendency to a lowering of the activation energy by deactivation is observed as has been the case in several of our earlier work [19, 29, 30].
Liu et al. [24] found that high phosphorus concentrations decrease the SCR rate by weakened NH3 storage capacity below 350 °C. An enhanced parasitic oxidation of NH3 above 400 °C is also observed on a deactivated catalyst as did we (see below).
We tried to correlate the measured rate constants to the amount of individual poison contents in the materials used for 890 h. The general trend was an exponential decrease, as found by Chen et al. [32], with the amount of poison (Fig. 10 for P). Similar trends were obtained for Zn, Ca and S. The scatter of data was too large to take this analysis any further.
Chen et al. [32] showed that P and Ca deactivated a 5% vanadia/TiO2 by 23% at Ca or P/V = 0.3 and 300 °C. Their rate constants were 10.5 for the fresh and 8.1 cm3/g/s for the deactivated catalyst. Ours were 5 × larger.
3.4 The Selectivity in the SCR of NO with NH3
It is clear from Fig. 11 that the amount of N2O formed in the SCR is much lower in the front of the catalyst where the catalyst is more poisoned. The longer the catalyst is used the more N2O is formed. For the fresh catalyst the measurements of N2O was only done on pieces 3 and 9. Here the formation of N2O was 66 and 65 ppm i.e. almost the same. This also holds for the total rates of reduction (Table 3) which becomes constant from piece 3 and further away from the inlet of the monolith. More N2O is formed after 890 h than after 2299 h. This is probably caused by the higher rates as this catalyst is less poisoned than the latter one. Yates et al. [30] studied the SCR of 1000 ppm NO with 1000 ppm NH3 with 3% O2 in Ar using a mass spectrometer for analysis. For a 10% V2O5/TiO2 catalyst the selectivity to N2O was 9.5% at a NO conversion of 83%. We use 200 ppm and 200 ppm NH3 and if we assume that there is a direct proportionality their outlet concentration of 79 ppm N2O recalculates to 16 ppm at our conditions. We experimentally determined the content of N2O to 2 and 3 ppm for the 3rd and 9th pieces. We used a 5.6% V2O5 catalyst and this content should lower the amount to about 8 ppm. There is a good agreement with the different studies especially if we consider the possibility of a more crystalline catalyst in the studies by Yates.
3.5 The Activity in the Oxidation of NH3 with O2
The conversion of NH3 increases with temperature above 340 °C as expected. The time of use does not seem to influence the conversion much (Fig. 12). Comparing again to Yates et al. [33] their conversion was 98% at 350 °C while we measured 95% for the fresh catalyst.
The reaction system for the SCR reactions including side reactions are shown below. These are all proposed in a paper by Djerad et al. [34] in a study of the effect of oxygen concentration on the SCR over V2O5 (3%)–WO3 (9%)/TiO2. They also used a coated catalyst on a 400 cpsi cordierite monolith for their study.
At low temperatures reactions 1 to 3 are dominating where reaction 1 is the warranted SCR reaction. The activation energies for all reactions increase with temperature in the order shown by the reaction number.
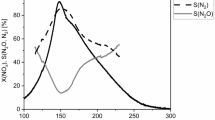
The products formed in the oxidation of NH3 at increasing temperatures are: N2, N2O and at high temperatures NO, and in all reactions also H2O. The reaction 2 gives N2 at low temperatures by direct oxidation of NH3 with O2. The selectivity for the formation of N2 in this reaction is shown in Fig. 13. The fresh catalyst has a higher yield of N2 than the poisoned ones. The more poisoned the catalyst is the lower amounts of N2 it produces. There is a trend of lower relative amounts of N2 at higher temperatures caused by other side reactions as described below. Yates et al. [33] reported a 23% selectivity to N2 at 350 °C. Our value was 95%.
One of the reactions forming N2O (reaction 3) is the NSR reaction (non-selective reduction of NO with NH3). N2O is also formed at higher temperatures by the direct oxidation of NH3 with O2 (reaction 4). The selectivity for the formation of N2O is shown in Fig. 14. There is more N2O formed at higher temperatures and on the deactivated catalysts than on the fresh one. The time of use does not influence the yield of N2O above 380 °C. At 460 °C the selectivity to N2O is 29.4%. Below 380 °C the lowest amount of N2O is obtained for the catalyst used for 2299 h (sel. 3.8%). Djerad et al. [34] also studied the oxidation of 500 ppm ammonia with 2–15% O2 from 150 to 500 °C. At 300 °C N2O started to form and at 420 °C their selectivity to N2O was 27.2% compared to ours of 10%. The N2O formed was almost independent on the oxygen concentration. They did not measure N2 but calculated it by mass balance. Anyway, similar trends were observed in both studies even if the catalyst in their case contained WO3 in addition to V2O5.
The fresh catalyst starts to form NO by reaction 5 above 400 °C but produces only 9 ppm from 700 ppm NH3 at 450 °C (Fig. 15). This gives a selectivity of only 2.4%. The more deactivated and thus more poisoned catalysts produce more NO. The catalyst used for 890 h produces 19.8 ppm NO and the one used for 2299 h 34 ppm NO (sel. 11.9%) Thus, the more poisoned catalyst shows higher NH3 oxidation rates. Djerad et al. [34] found that no NO was formed at 420 °C but about 16 ppm at 500 °C. This also resembles our results for the fresh catalyst.
Ozkan et al. [35] in a study of the catalytic oxidation of NH3 assumed that V2O5 catalysts had three sites for adsorption of NH3. These are depicted in Fig. 16.
The 1st specie (a) has long surface residence time and forms N2 and N2O by reactions 2, 3 and 4.
The 2nd one (b) is short lived and very reactive and gives rise to NO and H2O at high temperatures (reaction 5, See Fig. 15).
The 3rd type (c) is not active in the direct oxidation of NH3 but is the one active in the SCR reaction (reaction 1).
An analysis of the experimental data gave kinetic information for several reactions. The results from experimental data of the oxidation of ammonia are presented in Table 5.
The apparent activation energies, determined from Arrhenius plots of individual rates, increase in the order expected from the appearance of the products at increased temperatures. The formation of N2O and NO are both high temperature reactions with low values of the absolute reaction rates. From the perfect linear form of the Arrhenius plots it is clear that they are not influenced by diffusion processes. The formation of N2 on the other hand is probably so. In fact, the rate of NO formation at 340 °C is only 0.15% of the rate of N2 production. At 460 °C the ratio is 0.26%. The formation of N2O is larger and the values are 5.9 and 26.7% respectively.
There are similar trends for all values of the apparent activation energies. They all decrease when the catalyst gets more poisoned. We have shown this fact several times before [29, 30].
The pre-exponential factors show the decreased activity when the catalysts are deactivated.
4 Conclusions
The results from this study show that the fresh catalyst is not uniform but contains about 35% less active material in the 1st piece compared to the other ones. It is also shown that the test procedure as such decreases the surface areas considerably. Therefore, it is necessary to compared surface specific activities to get a full understanding of the effects of the deactivation. The further away from the poison laded inlet of the catalyst one comes the less effect has the poison on the structure of the catalyst. This hold for all catalysts studied.
The pore structure of the fresh catalyst changes to a structure with larger pores on time of use.
Many poisons, especially P, Ca, Zn and S were accumulated in the deactivated catalysts and preferentially in the 1st 6 mm from the inlet.
The results of this investigation show an increase in catalytic activity due to SO2 (as sulphates) up to 890 h on-stream, at which the maximum activity is reached. This is an important result, since on-stream activation actually prolongs the lifetime of the catalyst. Different pieces deactivate at different rates. The largest deactivation is observed for the 1st piece, which also contains the largest amounts of poisons and the smallest amount of active material. A correlation of the rate constant to the accumulated amount of poisons show a decreasing trend of activity for all materials and all poisoned considered. The scatter of these data is very large so more analysis is needed to clarify this effect.
The results presented here are important, not only for diesel SCR catalysts, but also for SCR catalysts in general, since they show how deactivation proceeds when compounds in the exhaust gases deactivate the catalyst in a monolith structure.
References
Konstandopoulos A, Zarvalis D, Chasapidis L, Deloglou L, Vlachos N, Kotbra A, Anderson G (2017) SAE Int J Engines 10(4):1653
Andersson SL, Gabrielsson PLT, Odenbrand CUI (1994) AIChE J 40:1911
Hanell B et al. (1996) Stiftelsen för Värmeteknisk Forskning, Stockholm, Nr. 558, 1996
Kamata H, Takahashi T, Odenbrand CUI (1998) Catal Lett 53:65
Odenbrand CUI (2008) Chem Eng Res Des 86(7):663
Jung H, Kittelson DB, Zachariah MR (2003) SAE Technical Paper 2003-01-3179
Nicosia D, Elsener M, Kröcher O, Jansohn P (2007) Top Catal 42–43:333
Kröcher O, Elsener M (2008) Appl Catal B 75:215
Nicosia D, Czekaj I, Kröcher O (2008) Appl Catal 95:39
Klimczak M, Kern P, Heinzelmann T, Lucas M, Claus P (2010) Appl Catal B 95:39
Maunula T, Kinnunen T, Iivonen M (2011) SAE Technical Paper 2011-01-1316
Pietikäinen M, Väliheikki A, Oravisjärvi K, Kolli T, Huutanen M, Niemi S, Virtanen S, Karhu T, Keiski R (2015) Renew Energy 77:377
Schobing J, Tchamber V, Brilhac JF, Auclaire A, Vonarb R (2016) Top Catal 59:1019
Castellino F, Degn Jensen A, Johnsson JE, Fehrmann R (2009) Appl Catal B 86:206
Cho CP, Pyo YD, Jang JY, Kim GC, Shin YJ (2017) Appl Therm Eng 110:18
Efstathiou AM, Fliatoura K (1995) Appl Catal B 6:35
Duffy BL, Curry-Hyde HE, Cant NW, Nelson PF (1994) J Phys Chem 98:7153
Ozkan US, Cai Y, Kumthekar MW, Zhang L (1993) J Catal 142:182
Odenbrand CUI (2018) Appl Catal B 234:365
Brunauer S, Emmett PH, Teller E (1938) J Am Chem Soc 60:309
Barett EP, Joyner LG, Halenda PP (1951) J Am Chem Soc 73:373
Harkins WD, Jura G (1944) J Am Chem Soc 66(6):919
Madia G, Elsener M, Koebel M, Raimondi F, Wokaun A (2002) Appl Catal B 39:181
Liu Y, Liu Z, Mnichowicz B, Harinath AV, Li H, Bahrami B (2016) Chem Eng J 287:680
https://digital.library.unt.edu/ark:/67531/metadc12748/m1/22/
Vaimakis TC, Pomonis PJ, Sdoukos AT (1990) Thermochim Acta 168:103
Sahimi M, Tsotsis TT (1985) J Catal 96:552
Tokarz M, Järås S, Persson B (1991) In: Bartholomew CH, Butt JB (eds) Catalyst Deactivation 1991. Elsevier, Amsterdam, p 523
Brandin JGM, Odenbrand CUI (2017) Top Catal 60:1306
Brandin JGM, Odenbrand CUI (2018) Catal Lett 148(1):312
Amiridis MD, Solar JP (1996) Ind Eng Chem Res 35:978
Chen JP, Buzanowski MA, Yang RT, Cichanowicz JE (1990) J Air Waste Manag Assoc 40:1404
Yates M, Martín JA, Martín-Luengo MA, Suaréz S, Blanco J (2005) Catal Today 107–108:120–125
Djerad S, Crocoll M, Kureti S, Tifouti L, Weisweler W (2006) Catal Today 113:208
Ozkan SU, Cai Y, Kuthekar MW (1994) J Catal 149:375
Acknowledgements
Open access funding provided by Lund University. We wish to thank Mr Martin Bruszt who measured the rate of reduction NOx. Mrs Birgitta Lindén helped us with the nitrogen adsorption measurements. The procurement of experimental data for this study was financed by the Swedish National Board for Industrial and Technological Development and their support is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Odenbrand, C.U.I. Penetration of Poisons Along the Monolith Length of a V2O5/TiO2 Diesel SCR Catalyst and Its Effect on Activity. Catal Lett 149, 3476–3490 (2019). https://doi.org/10.1007/s10562-019-02892-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02892-7