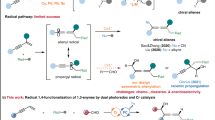
Abstract
Under photocatalytic reductive conditions, trifluoromethyl radical addition onto an ynamide followed by cyclization on a benzoyl moiety produces diverse isoindolinone platforms with good yields. The selectivity of the radical cyclization, N-benzoyl vs. N-benzyl as radical acceptor and the E/Z ratio of isomers have been rationalized by modeling.
Similar content being viewed by others
References
Albert M, Fensterbank L, Lacôte E, Malacria M. Tandem radical reactions. In: Gansäuer A, Ed. Topics Current Chemistry. Vol 264. Berlin: Springer, 2006. 1–62
Baralle A, Baroudi A, Daniel M, Fensterbank L, Goddard JP, Lacôte E, Larraufie MH, Maestri G, Malacria M, Ollivier C. Radical cascade reactions. In: Chatgilialoglu C, Studer A, Eds. Encyclopedia of Radicals in Chemistry, Biology and Materials. Chichester: John Wiley & Sons, 2012. 729–766
Godineau E, Landais Y. Chem Eur J, 2009, 15: 3044–3055
Liautard V, Landais Y. Free-radical multicomponent processes. In: Zhu J, Wang Q, Wang MX, Eds. Multicomponent Reactions. 2nd Ed. Weinheim: Wiley, 2014. 401–438
Chen JR, Yu XY, Xiao WJ. Synthesis, 2015, 47: 604–629
Zhang Y, Sun K, Lv Q, Chen X, Qu L, Yu B. Chin Chem Lett, 2019, 30: 1361–1368
Xuan J, Studer A. Chem Soc Rev, 2017, 46: 4329–4346
Huang HM, Garduño-Castro MH, Morrill C, Procter DJ. Chem Soc Rev, 2019, 48: 4626–4638
Dutta S, Mallick RK, Prasad R, Gandon V, Sahoo AK. Angew Chem Int Ed, 2019, 58: 2289–2294
Wang J, Sánchez-Roselló M, Aceña JL, del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H. Chem Rev, 2014, 114: 2432–2506
Dagousset G, Carboni A, Masson G, Magnier E. Visible light-induced (per)fluoroalkylation by photoredox catalysis. In: Groult H, Leroux FR, Tressaud A, Eds. Modern Synthesis Process and Reactivity of Fluorinated Compounds: Progress in Fluorine Science. Amsterdam: Elsevier, 2017. 389–426
Oh E, Kim H, Han S. Synthesis, 2018, 50: 3346–3358
Fuentes N, Kong W, Fernández-Sánchez L, Merino E, Nevado C. J Am Chem Soc, 2015, 137: 964–973
Zheng J, Deng Z, Zhang Y, Cui S. Adv Synth Catal, 2016, 358: 746–751
Li Y, Lu Y, Qiu G, Ding Q. Org Lett, 2014, 16: 4240–4243
Noto N, Miyazawa K, Koike T, Akita M. Org Lett, 2015, 17: 3710–3713
Banerjee B, Litvinov DN, Kang J, Bettale JD, Castle SL. Org Lett, 2010, 12: 2650–2652
Sato A, Yorimitsu H, Oshima K. Synlett, 2009: 28–31
Marion F, Courillon C, Malacria M. Org Lett, 2003, 5: 5095–5097
Marion F, Coulomb J, Servais A, Courillon C, Fensterbank L, Malacria M. Tetrahedron, 2006, 62: 3856–3871
Balieu S, Toutah K, Carro L, Chamoreau LM, Rousselière H, Courillon C. Tetrahedron Lett, 2011, 52: 2876–2880
Baguia H, Deldaele C, Romero E, Michelet B, Evano G. Synthesis, 2018, 50: 3022–3030
Wang CS, Dixneuf PH, Soulé JF. Chem Rev, 2018, 118: 7532–7585
Staveness D, Bosque I, Stephenson CRJ. Acc Chem Res, 2016, 49: 2295–2306
Shaw MH, Twilton J, MacMillan DWC. J Org Chem, 2016, 81: 6898–6926
Pawlowski R, Stanek F, Stodulski M. Molecules, 2019, 24: 1533–1566
Festa AA, Voskressensky LG, Van der Eycken EV. Chem Soc Rev, 2019, 48: 4401–4423
Chen JR, Hu XQ, Lu LQ, Xiao WJ. Acc Chem Res, 2016, 49: 1911–1923
Tanoury G. Synthesis, 2016, 48: 2009–2025
Cook AM, Wolf C. Tetrahedron Lett, 2015, 56: 2377–2392
Evano G, Coste A, Jouvin K. Angew Chem Int Ed, 2010, 49: 2840–2859
Zhang Y, Hsung RP, Tracey MR, Kurtz KCM, Vera EL. Org Lett, 2004, 6: 1151–1154
Charpentier J, Früh N, Togni A. Chem Rev, 2015, 115: 650–682
Eisenberger P, Gischig S, Togni A. Chem Eur J, 2006, 12: 2579–2586
For trifluoromethyl-containing compounds redox potentials: Jiang Y, Yu H, Fu Y, Liu L. Sci China Chem, 2015, 58: 673–683
For photocatalysts redox potentials: Prier CK, Rankic DA, MacMillan DWC. Chem Rev, 2013, 113: 5322–5363
For first uses in photoredox catalysis, see: Luo J, Zhang J. ACS Catal, 2016, 6: 873–877
For first uses in photoredox catalysis, see: Lévêque C, Chenneberg L, Corcé V, Ollivier C, Fensterbank L. Chem Commun, 2016, 52: 9877–9880
For a review, see: Shang TY, Lu LH, Cao Z, Liu Y, He WM, Yu B. Chem Commun, 2019, 55: 5408–5419
For a revision of the redox potentials, see: Le Vaillant F, Garreau M, Nicolai S, Gryn’ova G, Corminboeuf C, Waser J. Chem Sci, 2018, 9: 5883–5889
Jacquet J, Blanchard S, Derat E, Desage-El Murr M, Fensterbank L. Chem Sci, 2016, 7: 2030–2036
Singh K, Staig SJ, Weaver JD. J Am Chem Soc, 2014, 136: 5275–5278
Lin QY, Xu XH, Qing FL. J Org Chem, 2014, 79: 10434–10446
Larraufie MH, Courillon C, Ollivier C, Lacote E, Malacria M, Fensterbank L. J Am Chem Soc, 2010, 132: 4381–4387
Bogen S, Gulea M, Fensterbank L, Malacria M. J Org Chem, 1999, 64: 4920–4925
Acknowledgements
We thank Sorbonne Université, CNRS and Servier for funding. The authors wish to acknowledge the analytical department of IDRS — Servier for the compounds analyses (IR, NMR, HR-MS) and the SRIMC department for the syntheses on big scale. This work was granted access to the high performance computing (HPC) resources of the HPCaVe Centre at Sorbonne Université and to the HPC resources of IDRIS under the allocation 2018-A0050810312 made by GENCI. The authors wish to acknowledge support from the ICMG Chemistry Nanobio Platform-PCECIC, Grenoble, for calculations facilities. Jérémy Forté is acknowledged for the X-ray diffraction analyses as well as Conor Dent Cullen and Scott Warchal for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cassé, M., Nisole, C., Dossmann, H. et al. Trifluoromethyl radical triggered radical cyclization of N-benzoyl ynamides leading to isoindolinones. Sci. China Chem. 62, 1542–1546 (2019). https://doi.org/10.1007/s11426-019-9627-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-019-9627-x