Abstract
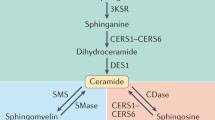
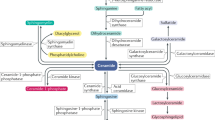
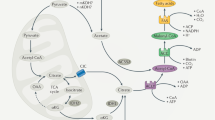
Ceramides are products of metabolism that accumulate in individuals with obesity or dyslipidaemia and alter cellular processes in response to fuel surplus. Their actions, when prolonged, elicit the tissue dysfunction that underlies diabetes and heart disease. Here, we review the history of research on these enigmatic molecules, exploring their discovery and mechanisms of action, the evolutionary pressures that have given them their unique attributes and the potential of ceramide-reduction therapies as treatments for cardiometabolic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Holland, W. L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Chaurasia, B. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392 (2019).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Kurek, K. et al. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 34, 1074–1083 (2014).
Correnti, J. M., Juskeviciute, E., Swarup, A. & Hoek, J. B. Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G959–G973 (2014).
Kasumov, T. et al. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One 10, e0126910 (2015).
Chen, T. C. et al. An ANGPTL4-ceramide-PKCzeta axis mediates chronic glucocorticoid exposure-induced hepatic steatosis and hypertriglyceridemia in mice. J. Biol. Chem. 294, 9213–9224 (2019).
Dekker, M. J. et al. Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis 228, 98–109 (2013).
Park, T. S., Rosebury, W., Kindt, E. K., Kowala, M. C. & Panek, R. L. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 58, 45–51 (2008).
Glaros, E. N. et al. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res. 49, 324–331 (2008).
Glaros, E. N. et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol. 73, 1340–1346 (2007).
Park, T. S. et al. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 189, 264–272 (2006).
Hojjati, M. R. et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 280, 10284–10289 (2005).
Park, T. S. et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110, 3465–3471 (2004).
Park, T. S. et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112 (2008).
Ji, R. et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2, 82922 (2017).
Holland, W. L. et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121, 1858–1870 (2011).
Vasiliauskaité-Brooks, I. et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017).
Westra, B. Ceramides, Plasma [A Test in Focus]. Mayo Clinic Laboratories https://news.mayomedicallaboratories.com/2016/07/28/ceramides-plasma-a-test-in-focus/ (2016).
Summers, S. A. Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 (2018).
Merrill, A. H. Jr. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846 (2002).
Park, J. W., Park, W. J. & Futerman, A. H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 1841, 671–681 (2014).
Hannun, Y. A. & Obeid, L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem. Sci. 20, 73–77 (1995).
Kolesnick, R. Ceramide: a novel second messenger. Trends Cell Biol. 2, 232–236 (1992).
Obeid, L. M., Linardic, C. M., Karolak, L. A. & Hannun, Y. A. Programmed cell death induced by ceramide. Science 259, 1769–1771 (1993).
Shimabukuro, M. et al. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats: role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273, 32487–32490 (1998).
Shimabukuro, M., Zhou, Y. T., Levi, M. & Unger, R. H. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl Acad. Sci. USA 95, 2498–2502 (1998).
Summers, S. A., Garza, L. A., Zhou, H. & Birnbaum, M. J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18, 5457–5464 (1998).
Wang, C. N., O’Brien, L. & Brindley, D. N. Effects of cell-permeable ceramides and tumor necrosis factor-alpha on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes 47, 24–31 (1998).
Zhou, H., Summers, S. A., Birnbaum, M. J. & Pittman, R. N. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 273, 16568–16575 (1998).
Jay, A. G. & Hamilton, J. A. The enigmatic membrane fatty acid transporter CD36: new insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot. Essent. Fat. Acids 138, 64–70 (2018).
Xu, S., Jay, A., Brunaldi, K., Huang, N. & Hamilton, J. A. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry 52, 7254–7261 (2013).
Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).
Hyde, R., Hajduch, E., Powell, D. J., Taylor, P. M. & Hundal, H. S. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 19, 461–463 (2005).
Finicle, B. T. et al. Sphingolipids inhibit endosomal recycling of nutrient transporters by inactivating ARF6. J. Cell Sci. 131, jcs213314 (2018).
Guenther, G. G. et al. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl Acad. Sci. USA 105, 17402–17407 (2008).
Edinger, A. L. Starvation in the midst of plenty: making sense of ceramide-induced autophagy by analysing nutrient transporter expression. Biochem. Soc. Trans. 37, 253–258 (2009).
Cowart, L. A. & Obeid, L. M. Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim. Biophys. Acta 1771, 421–431 (2007).
Chung, N., Mao, C., Heitman, J., Hannun, Y. A. & Obeid, L. M. Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J. Biol. Chem. 276, 35614–35621 (2001).
Kogot-Levin, A. & Saada, A. Ceramide and the mitochondrial respiratory chain. Biochimie 100, 88–94 (2014).
Zigdon, H. et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 288, 4947–4956 (2013).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Hajduch, E. et al. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J. 410, 369–379 (2008).
Powell, D. J., Hajduch, E., Kular, G. & Hundal, H. S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 23, 7794–7808 (2003).
Bourbon, N. A., Sandirasegarane, L. & Kester, M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem. 277, 3286–3292 (2002).
Xia, J. Y. et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278 (2015).
Taniguchi, C. M. et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 3, 343–353 (2006).
Blouin, C. M. et al. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59, 600–610 (2010).
Stratford, S., Hoehn, K. L., Liu, F. & Summers, S. A. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279, 36608–36615 (2004).
Chavez, J. A. & Summers, S. A. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 419, 101–109 (2003).
Russo, S. B. et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122, 3919–3930 (2012).
Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta 1817, 1833–1838 (2012).
Hammerschmidt, P. et al. CerS6-derived sphingolipids interact with MFF and promote mitochondrial fragmentation in obesity. Cell 177, 1536–1552.e1523 (2019).
Smith, M. E. et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem. J. 456, 427–439 (2013).
Ehehalt, R. et al. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts). BMC Cell Biol. 9, 45 (2008).
Pohl, J. et al. Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry 43, 4179–4187 (2004).
Covey, S. D. et al. Cholesterol depletion inhibits fatty acid uptake without affecting CD36 or caveolin-1 distribution in adipocytes. Biochem. Biophys. Res. Commun. 355, 67–71 (2007).
Chaurasia, B., Holland, W. L. & Summers, S. A. Does this Schlank make me look fat? Trends Endocrinol. Metab. 29, 597–599 (2018).
Sociale, M. et al. Ceramide synthase Schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Rep. 22, 967–978 (2018).
Marra, F. & Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 68, 280–295 (2018).
Alkhouri, N., Dixon, L. J. & Feldstein, A. E. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 3, 445–451 (2009).
Hoekstra, D. Ceramide-mediated apoptosis of hepatocytes in vivo: a matter of the nucleus? J. Hepatol. 31, 161–164 (1999).
Nikolova-Karakashian, M. Sphingolipids at the crossroads of NAFLD and senescence. Adv. Cancer Res. 140, 155–190 (2018).
Garcia-Ruiz, C., Marí, M., Colell, A., Morales, A. & Fernandez-Checa, J. C. Metabolic therapy: lessons from liver diseases. Curr. Pharm. Des. 17, 3933–3944 (2011).
Brown, M. S. & Goldstein, J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Ussher, J. R. et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464 (2010).
Yang, G. et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297, E211–E224 (2009).
Blachnio-Zabielska, A. U. et al. Inhibition of ceramide de novo synthesis affects adipocytokine secretion and improves systemic and adipose tissue insulin sensitivity. Int. J. Mol. Sci. 19, E3995 (2018).
Zhang, Q. J. et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61, 1848–1859 (2012).
Chaurasia, B. et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24, 820–834 (2016).
Turpin-Nolan, S. M. et al. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 26, 1–10.e17 (2019).
Stancevic, B. & Kolesnick, R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 584, 1728–1740 (2010).
Mantovani, A. et al. Association of plasma ceramides with myocardial perfusion in patients with coronary artery disease undergoing stress myocardial perfusion scintigraphy. Arterioscler. Thromb. Vasc. Biol. 38, 2854–2861 (2018).
Anroedh, S. et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 59, 1729–1737 (2018).
Hilvo, M. et al. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 269, 159–165 (2018).
Havulinna, A. S. et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 36, 2424–2430 (2016).
Laaksonen, R. et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 (2016).
Tarasov, K. et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99, E45–E52 (2014).
Cheng, J. M. et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 243, 560–566 (2015).
Yu, J. et al. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can. J. Cardiol. 31, 357–363 (2015).
Pan, W. et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis. 25, 230–235 (2014).
Lemaitre, R. N. et al. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the Strong Heart Family Study. Diabetes 67, 1663–1672 (2018).
Jensen, P. N. et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the Strong Heart Family Study. EBioMedicine 41, 44–49 (2019).
Lemaitre, R. N. et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-b: the Strong Heart Family Study. Diabetes 67, 1663–1672 (2018).
Bergman, B. C. et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 309, E398–E408 (2015).
Haus, J. M. et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343 (2009).
Boon, J. et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62, 401–410 (2013).
Lopez, X., Goldfine, A. B., Holland, W. L., Gordillo, R. & Scherer, P. E. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab. 26, 995–998 (2013).
Brozinick, J. T. et al. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. (Lond.) 37, 1064–1070 (2013).
Warshauer, J. T. et al. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab. Res. Rev. 31, 734–744 (2015).
Wigger, L. et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 18, 2269–2279 (2017).
Luukkonen, P. K. et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 64, 1167–1175 (2016).
Luukkonen, P. K. et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 41, 1732–1739 (2018).
Amati, F. et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60, 2588–2597 (2011).
Coen, P. M. et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59, 80–88 (2010).
Coen, P. M. & Goodpaster, B. H. Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 23, 391–398 (2012).
Coen, P. M. et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severeobesity. Obes. (Silver Spring) 21, 2362–2371 (2013).
Dubé, J. J. et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54, 1147–1156 (2011).
Adams, J. M. II et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53, 25–31 (2004).
Othman, A. et al. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 3, e000073 (2015).
Othman, A. et al. Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia 55, 421–431 (2012).
Mwinyi, J. et al. Plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS One 12, e0175776 (2017).
Khan, A. & Hornemann, T. Correlation of the plasma sphingoid base profile with results from oral glucose tolerance tests in gestational diabetes mellitus. EXCLI J. 16, 497–509 (2017).
Bertea, M. et al. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 9, 84 (2010).
Russo, S. B., Tidhar, R., Futerman, A. H. & Cowart, L. A. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem. 288, 13397–13409 (2013).
Skovbro, M. et al. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51, 1253–1260 (2008).
Petersen, M. C. & Shulman, G. I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci. 38, 649–665 (2017).
Itani, S. I., Ruderman, N. B., Schmieder, F. & Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-alpha. Diabetes 51, 2005–2011 (2002).
Szendroedi, J. et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111, 9597–9602 (2014).
Nowotny, B. et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 62, 2240–2248 (2013).
Petersen, M. C. & Jurczak, M. J. CrossTalk opposing view: intramyocellular ceramide accumulation does not modulate insulin resistance. J. Physiol. (Lond.) 594, 3171–3174 (2016).
Summers, S. A. & Goodpaster, B. H. CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol. (Lond.) 594, 3167–3170 (2016).
Holland, W. L. & Scherer, P. E. PAQRs: a counteracting force to ceramides? Mol. Pharmacol. 75, 740–743 (2009).
Blachnio-Zabielska, A. U., Koutsari, C., Tchkonia, T. & Jensen, M. D. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obes. (Silver Spring) 20, 2341–2347 (2012).
de Mello, V. D. et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 52, 2612–2615 (2009).
Sims, K. et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 285, 38568–38579 (2010).
Schilling, J. D. et al. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem. 288, 2923–2932 (2013).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Kim, J. K. et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Invest. 108, 437–446 (2001).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Majumdar, I. & Mastrandrea, L. D. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine 41, 442–449 (2012).
Gonzalez, F. J., Jiang, C., Xie, C. & Patterson, A. D. Intestinal farnesoid X receptor signalling modulates metabolic disease. Dig. Dis. 35, 178–184 (2017).
Xie, C. et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017).
Gonzalez, F. J., Jiang, C. & Patterson, A. D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151, 845–859 (2016).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Curran, J. E. et al. 63rd Annual Meeting Am. Soc. Hum. Genet. https://www.ashg.org/2013meeting/abstracts/fulltext/f130122450.htm (2013).
Acknowledgements
The authors receive research support from the National Institutes of Health (DK112826 and DK108833 to W.L.H. and DK115824 and DK116450 to S.A.S.), the Juvenile Diabetes Research Foundation (JDRF 3-SRA-2019-768-A-B to W.L.H.), the American Diabetes Association (to S.A.S.), the American Heart Association (to S.A.S.), the Margolis Foundation (to S.A.S.) and the USDA (2019-67018-29250 to B.C.). B.C. received a pilot grant from the Diabetes Research Center at Washington University in St. Louis from the NIH under award number P30DK020579.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.A.S. is a co-founder and consultant for Centaurus Therapeutics.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Rights and permissions
About this article
Cite this article
Summers, S.A., Chaurasia, B. & Holland, W.L. Metabolic Messengers: ceramides. Nat Metab 1, 1051–1058 (2019). https://doi.org/10.1038/s42255-019-0134-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0134-8
This article is cited by
-
Gut microbiota affects the estrus return of sows by regulating the metabolism of sex steroid hormones
Journal of Animal Science and Biotechnology (2023)
-
Writing and erasing ceramides to alter liver disease
Nature Metabolism (2023)
-
CerS6-dependent ceramide synthesis in hypothalamic neurons promotes ER/mitochondrial stress and impairs glucose homeostasis in obese mice
Nature Communications (2023)
-
Regulation of serine palmitoyl-transferase and Rac1–Nox2 signaling in diabetic retinopathy
Scientific Reports (2022)
-
Genome–microbiome interplay provides insight into the determinants of the human blood metabolome
Nature Metabolism (2022)