Abstract
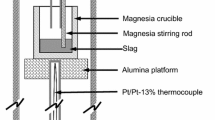
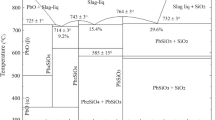
Experiments were performed using a range of test conditions to elucidate the rate controlling step during the reaction of liquid iron-carbon droplets and slags containing manganese oxide. Four conditions were tested in the system: initial MnO content in the slag (5, 10, and 15 wt pct), initial carbon content of the metal (1, 2.5, 4.3 wt pct), initial droplet mass (0.5, 1.0, and 1.5 g), and reaction temperature (1823 K [1550 °C], 1873 K [1600 °C], and 1923 K [1650 °C]). Data were collected using the Constant Volume Pressure Increase (CVPI) technique which tracked the continuous pressure increase in the sealed furnace over time. Samples were quenched at the end of each experiment and chemistry was checked using LECO Carbon Analysis and ICP (Inductively Coupled Plasma) for manganese. The rate of reaction can be broken into a faster initial period related to internal CO formation, and a slower second reaction controlled by a complex mechanism involving transport of oxygen from slag to metal via CO2 and decomposition of the CO2 at the gas–metal interface.


















Similar content being viewed by others
References
B.J. Jamieson and K.S. Coley: Metall. Mater. Trans. B, 2017, vol. 48, pp. 1613–24.
B.J. Jamieson, Y. Tabatabaei, M. Barati, and K. S. Coley: Metall. Mater. Trans. B, 2019, vol. 50, pp. 192–203.
R. Elliott, K. Coley, S. Mostaghel, and M. Barati: Jom, 2018, vol. 70, pp. 1–11.
O.S. Bobkova and V.V. Barsegyan: Metallurgist, 2006, vol. 50, pp. 463–68.
L.N. Kologrivova, A.Ya. Nakonechnyi, Z.G. Trofimova, O.V. Nosochenko, and N.N. Kulik: Metallurg, 1987, vol. 5, pp. 28–29.
O.I. Nokhrina, V.P. Komshukov, and V.I. Dmitrienko: Metallurgist, 2004, vol. 48, pp. 264–65.
M. Eissa, H. El-Faramawy, and G. Farid: Steel Res., 1998, vol. 69, pp. 373–80.
W.L. Daines and R.D. Pehlke: Metall. Trans., 1971, vol. 2, pp. 1203–11.
I.D. Sommerville, P. Grieveson, and J. Taylor: Ironmak. Steelmak., 1980, vol. 7, pp. 25–32.
P. Wei, M. Sano, M. Hirasawa, and K. Mori: ISIJ Int., 1991, vol. 31, pp. 358–65.
K. Xu, G. Jiang, W. Ding, L. Gu, S. Guo, and B. Zhao: ISIJ Int., 1993, vol. 33, pp. 104–8.
S.K. Tarby and W.O. Philbrook: Trans. Metall. Soc. AIME, 1967, vol. 239, pp. 1005–17.
T. Yagi and Y. Ono: Trans. Iron Steel Inst. Japan, 1970, vol. 10, pp. 36–37.
R.J. Pomfret and P. Grieveson: Ironmak. Steelmak., 1978, vol. 5, pp. 191–97.
Y. Kawai, N. Shinozaki, and K. Mori: Can. Metall. Q., 1982, vol. 21, pp. 385–91.
N. Shinozaki, K. Ishido, K. Mori, and Y. Kawai: Tetsu-to-Hagane, 1984, vol. 70, pp. 73–80.
H. Sohn, Z. Chen, and W. Jung: Steel Res., 2000, vol. 71, pp. 145–52.
M.A. Rhamdhani: PhD Thesis—McMaster Univ., 2005.
C.L. Molloseau, and R.J. Fruehan: Metall. Mater. Trans. B. 2002, vol. 33, pp. 335–44.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B 2017, vol. 48, pp. 2984–3001.
E. Chen and K.S. Coley: Ironmak. Steelmak., 2010, vol. 37, pp. 541–45.
D.R. Sain and G.R. Belton: Metall. Trans. B, 1976, vol. 7, pp. 235–44.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B, 2018, vol. 49, pp. 1119–35.
D. J. Min and R. J. Fruehan: Metall. Trans. B, 1992, vol. 23, pp. 29–37.
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, and M.A. Van Ende: Calphad, 2016, vol. 54, pp. 35–53.
P. Wei, M. Sano, M. Hirasawa, and K. Mori: Trans. Iron Steel Inst. Japan, 1988, vol. 28, pp. 637–44.
Acknowledgements
The authors thank the National Science and Research Council of Canada (NSERC, STPGP463252-14) for funding support. Special thanks to ArcelorMittal Dofasco, Stelco, Praxair, and Hatch Ltd. for their in-kind support, technical expertise, and their many helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted February 19 2019.
Rights and permissions
About this article
Cite this article
Jamieson, B.J., Barati, M. & Coley, K.S. Kinetics of the Carbothermic Reduction of Manganese Oxide from Slag. Metall Mater Trans B 50, 2733–2746 (2019). https://doi.org/10.1007/s11663-019-01696-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01696-9