Abstract:
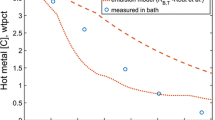
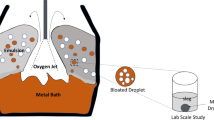
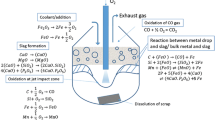
A mathematical model has been developed to predict the decarburization rate within individual droplets in the emulsion zone. All the chronological events pertaining to the life cycle of a metal droplet in the emulsion zone like oxygen supply (from slag), external and internal decarburization have been modeled dynamically and validated against experimental data available in open literature. The bloating behavior of metal droplets in the emulsion was represented theoretically by incorporating an escape function dependent on internal CO gas generation. The model is able to predict the onset of bloating and the residence time of metal droplets in the emulsion zone. The residence time of droplet containing 2.6 wt pct C and 0.007 wt pct S is in the range of 10 to 13 seconds. The contribution of decarburization rate in the emulsion zone to the overall decarburization rate is studied using the industrial data reported by Cicutti et al. The model predicts 5 to 75 pct of total decarburization takes place in the emulsion zone. It is found that the extent of decarburization of a metal droplet depends on its initial carbon content rather than its oxygen content for slag containing FeO greater than 10 wt pct.











Similar content being viewed by others
Abbreviations
- \( A_{\text{d}} \) :
-
Surface area of a metal droplet, \( \left( {{\text{m}}^{2} } \right) \)
- \( a_{\text{FeO}} \) :
-
Raoultian activity of FeO in slag \( \left( - \right) \)
- \( e_{j}^{i} \) :
-
First order interaction parameter, of solute \( j \) on \( i \)
- \( f_{\text{C}} ,\,f_{\text{O}} , \,f_{\text{S}} \) :
-
Henrian activity coefficients of carbon, oxygen, and sulfur, respectively
- \( h_{\text{C}} ,\,\,h_{\text{O}} , \,h_{\text{S}} \) :
-
Henrian activities of carbon, oxygen, and sulfur, respectively
- \( J_{\text{C}} , \,J_{\text{FeO}} , \,J_{\text{O}} \) :
-
Flux of carbon, oxygen, and FeO towards metal droplet–slag surface, \( \left(\frac{{\text{moles}}}{{\text{s}}}\right) \)
- \( J_{\text{ext}} , \,J_{\text{int}} \) :
-
External and internal CO generation rates respectively, \(\left(\frac{{\text{moles}}}{{\text{s}}}\right) \)
- \( J_{\text{esc}} \) :
-
Net escaped moles of CO gas at a given time step, \( \left( {\text{moles}} \right) \)
- \( J_s \) :
-
Nucleation rate, \( \left( \frac{\text{nuclei}}{{{\text{m}}^{3} \cdot {\text{s}}}} \right) \)
- \( K_{\text{CO}} , K_{\text{FeO}} \) :
-
Equilibrium constants for CO formation and FeO dissociation reaction
- \( K_{\text{O}} , \,K_{\text{S}} \) :
-
Adsorption coefficients of the oxygen and sulfur \( \left( - \right) \)
- \( k_{\text{s}} , k_{\text{m}} \) :
-
Slag and metal phase mass transfer coefficient, \( \left( \frac{{\text{m}}}{{\text{s}}}\right) \)
- \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {k} , \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\leftharpoonup}$}} {k} \) :
-
Forward and backward reaction constants of FeO dissociation reaction \( \left( - \right) \)
- \( m_{\text{d}} \) :
-
Mass of metal droplet, \( \left( {\text{kg}} \right) \)
- \( N_{\text{CO}} \) :
-
Net retained moles of CO gas within a metal droplet, \( \left( {\text{moles}} \right) \)
- \( P_{\text{CO}}^{\text{ext}} , \Delta P_{\text{CO}}^{\text{int}} \) :
-
Supersaturation pressure for external (droplet surface) and internal CO gas generation (atm)
- \( t_{\text{inst}} , \,t_{\text{res}} , \,\Delta t \) :
-
Instantaneous time, residence time, time step, respectively \( \left( {\text{seconds}} \right) \)
- \( \Delta t_{\text{e}} \) :
-
Time interval during which the emulsion zone decarburization is being evaluated
- \( V_{o} \) :
-
Initial volume of metal droplet, \( \left( {{\text{m}}^{ 3} } \right) \)
- \( \theta_{\text{S}} \) :
-
Fraction of surface area poisoned by sulfur \( \left( - \right) \)
- \( \rho_{t} , \rho_{\text{d}} \) :
-
Apparent and initial density of metal droplet \( \left( \frac{\text{kg}}{{{\text{m}}^{3} }} \right) \)
- \( \rho_{\text{g}} , \rho_{\text{s}} ,\rho_{{{\text{s}} - {\text{g}}}} \) :
-
Densities of gas, slag, and emulsion, respectively \( \left( \frac{\text{kg}}{{{\text{m}}^{3} }} \right) \)
- \( \sigma , \sigma_{\text{metal}} \) :
-
Surface tension of metal, \(\left({\frac{{\text{N}}}{{\text{m}}}}\right) \)
- \( \phi_{\text{g}} \) :
-
Volume fraction of CO gas in the emulsion
- \( \psi \) :
-
Correction factor to the surface tension of the metal \( \left( - \right) \)
- r :
-
Slag–metal interface
- s − g :
-
Emulsion
References
H.W. Meyer, W.F. Porter, G. Smith, and J. Szekely: J. Met., 1968, vol. 20, pp. 35–42.
J. Schoop, W. Resch, and G. Mahn: Ironmak. Steelmak., 1978, vol. 2, pp. 72–9.
R.C. Urquhart and W.G. Davenport: Can. Metall. Q., 1973, vol. 12, pp. 507–16.
P. Kozakevitch: J. Miner. Met. Metarials Soc., 1969, vol. 22, pp. 57–68.
D.J. Price: in Process Engineering of Pyrometallurgy Symposium, The Institution of Mining and Metallurgy, London, 1974, pp. 8–15.
C. Cicutti, M. Valdez, T. Pérez, R. Donayo, and J. Petroni: Lat. Am. Appl. Res., 2002, vol. 32, pp. 237–40.
E.W. Mulholland, G.S. Hazeldean, and M. Davies: J. Iron Steel Inst., 1973, vol. 211, pp. 632–39.
T. Gare and G.S.. Hazeldean: Ironmak. Steelmak., 1981, vol. 4, pp. 169–81.
H. Gaye and Riboud.P.V: Metall. Trans. B, 1977, vol. 8, pp. 409–15.
D.J. Min and R.J. Fruehan: Metall. Trans. B, 1992, vol. 23, pp. 29–37.
C.L. Molloseau and R.J. Fruehan: Metall. Mater. Trans. B, 2002, vol. 33, pp. 335–44.
E. Chen: Ph.D Thesis, McMaster University, 2011.
E. Chen and K.S. Coley: Ironmak. Steelmak., 2010, vol. 37, pp. 541–5.
M.D. Pomeroy: MASc Thesis, McMaster University, 2011.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B 2017, 48, 1–18.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B, 2017, vol. 48, pp. 2343–53.
K.S. Coley, E. Chen, and M. Pomeroy: in Proceedings of the Extraction and Processing Division Symposium on Pyrometallurgy in Honor of David G.C. Robertson, P.J. Mackey, E.J. Grimsey, R.T. Jones, and G.A. Brooks, eds., 2014, pp. 289–302.
H. Sun and G. Zhang: in 3rd International Congress on Science ans Technology of Steelmaking, 2005, pp. 257–68.
N. Dogan, G.A. Brooks, and M.A. Rhamdhani: ISIJ Int., 2011, vol. 51, pp. 1093–101.
N. Dogan: Ph.D Thesis, Swinburne University, 2011.
R. Sarkar, P. Gupta, S. Basu, and N.B. Ballal: Metall. Mater. Trans. B, 2015, vol. 46, pp. 961–76.
B. Rout, G. Brooks, M.A. Rhamdhani, Z. Li, F.N. Schrama, and J. Sun: Metall. Mater. Trans. B, 2018, vol. 49, pp. 537–57.
B.K. Rout, G. Brooks, M. Akbar Rhamdhani, Z. Li, F.N.H. Schrama, and A. Overbosch: Metall. Mater. Trans. B, 2018, 49, 1022–33.
G. Brooks, Y. Pan, and K.S. Coley: Metall. Mater. Trans. B, 2005, 36, 525–35.
Subagyo, G. A. Brooks, K.S. Coley, and G. A. Irons: ISIJ Int., 2003, 43, 983–89.
G.R. Belton: Met. Trans. B, 1976, 7, 35–42.
Y. Kawai and Y. Shiraishi: Handbook of Physico-Chemical Properties at High Temperatures. Iron and Steel Institute of Japan, Tokyo 1988.
M. Hino and K. Ito, eds.: Thermodynamic Data for Steelmaking, Tohoku University Press, Sendai, 2010.
G.K. Sigworth and J.F. Elliot: Met. Sci., 1974, vol. 3, pp. 298–310.
E. Shibata, H. Sun, and K. Mori: Metall. Mater. Trans. B 1999, 30, 279–86.
L.A. Baker, N.A. Warner, and A.E. Jenkins: Trans. Metall. Soc. AIME, 1967, vol. 239, pp. 857–64.
P.A.A. Distin, G.D.D. Hallett, and F. D. Richardson: J. Iron Steel Inst., 1968, 1, 821–33.
N. El Kaddah and D.G.. Robertson: J. Colloid Interface Sci., 1977, vol. 60, pp. 349–60.
K. Mor, H. Sun, K. Ga, V. Sahajwalla, and R.D. Pehlke: ISIJ Int., 1999, vol. 39, pp. 25–33.
K. Gao, V. Sahajwalla, H. Sun, C. Wheatley, and R. Dry: ISIJ Int., 2000, vol. 40, pp. 301–8.
H.S. Levine: Metall. Trans. B, 1973, vol. 4, pp. 777–82.
P.G. Bowers, K. Bar-eli, and R.M. Noyes: J. Chem. Soc. Faraday Trans., 1996, vol. 92, pp. 2843–9.
S.D. Lubetkin: Langmuir, 2003, vol. 19, pp. 2575–87.
A.W. Cramb, and I. Jimbo: Steel Res., 1989, 60, 157–65.
W. Cramb, W.R. Graham, and G.R. Belton: Metall. Trans. B. 9, 623–629 (1978)
K. Ogino, S. Hara, T. Miwa, and S. Kimoto: Trans. ISIJ, 1984, vol. 24, pp. 522–31.
R. Hongbin, M. Suzuk, D.R. Poirier, H. Yin, M. Suzuki, and T. Emi: ISIJ Int., 1998, vol. 38, pp. 229–38.
P. Sahoo, T. Debroy, and M.J. McNallan: Metall. Trans. B, 1988, vol. 19, pp. 483–91.
Y. Chung and A.W. Cramb: Metall. Mater. Trans. B, 2000, vol. 31, pp. 957–71.
F.A. Halden and W.D. Kingery: J. Phys. Chem., 1955, vol. 59, pp. 557–9.
K.S. Coley and T.X. Zhu: Private Communication, Hamilton, Ontario, 2018.
Subagyo A., and G.A. Brooks: ISIJ Int., 2002, vol. 42, pp. 1182–4.
C. Cicutti, M. Valdez, T. Perez, J. Petroni, A. Gomez, R. Donayo, and L. Ferro: in 6th International Conference on Molten Slags, Fluxes and Salts, Stockholm- Helsinki, 2000, p. 367.
N. Dogan, G.A. Brooks, and M.A. Rhamdhani: ISIJ Int., 2011, vol. 51, pp. 1086–92.
P. Wei, M. Sano, M. Hirasawa, and K. Mori: ISIJ Int., 1993, vol. 33, pp. 479–87.
M. Barati and K.S. Coley: Metall. Mater. Trans. B, 2006, vol. 37, pp. 41–49.
M.S. Millman, A. Kapilashrami, M. Bramming, and D. Malmberg: Imphos : Improving Phosphorus Refining. European Union, Luxemborg, 2011.
B. Deo and R. Boom: Fundamentals of Steel Making Metallurgy. Pretince Hall International, Upper Saddle River (1993).
B.K. Rout, G. Brooks, M.A. Rhamdhani, and Z. Li: Metall. Mater. Trans. B, 2016, 47, 3350–61.
K. Koch, J. Falkus, and R. Bruckhaus: Steel Res. Int., 1993, vol. 64, pp. 15–21.
Acknowledgments
The authors would like to thank Prof. Kenneth Coley, Dr. Kezhuan Gu, and Mr. Tai Xi Zhu for fruitful discussions related to bloated droplet theory. This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), Project Number 20007117 and the McMaster Steel Research Centre (SRC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted March 26, 2019.
Appendix A
Appendix A
Calculation of Interfacial Activities of Metal Droplet Components
The activity coefficients for carbon and oxygen can be calculated by taking into account the interaction parameters of carbon, oxygen, and sulfur.
The Henrian activities of carbon and oxygen are defined by,
Evaluation of Mole Fraction and Activity of (FeO)
The mole fraction of FeO at the droplet–slag interface is defined by the following equation
The Raoultian activity of FeO at the interface can be defined by,
Balance of Supply and Consumption of Oxygen at the Interface
The equilibrium constant for decarburization reaction is defined as
The value of \( P_{\text{CO}}^{\text{ext}} \) is taken to be equal to 1.5 atm (equal to the pressure of the furnace).
The Eqs. [A7] and [A8] can be rearranged in terms of \( h_{\text{O}}^{r} \) and combined to arrive at the following equation
Rights and permissions
About this article
Cite this article
Kadrolkar, A., Dogan, N. The Decarburization Kinetics of Metal Droplets in Emulsion Zone. Metall Mater Trans B 50, 2912–2929 (2019). https://doi.org/10.1007/s11663-019-01710-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-019-01710-0