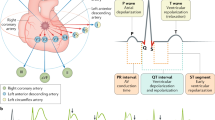
Abstract
Atherosclerosis and plaque disruption have a central pathological role in the majority of patients who present with an acute coronary syndrome (ACS), but non-atherosclerotic processes are also important contributors to a substantial number of ACS events and require different diagnostic and therapeutic strategies. In the absence of obstructive coronary artery disease, intravascular imaging techniques might be needed to delineate the underlying aetiology, together with a high index of suspicion for other important causes of ACS. In this Review, we discuss five non-atherosclerotic causes of ACS, including spontaneous coronary artery dissection, coronary artery embolism, vasospasm, myocardial bridging and stress-induced cardiomyopathy (Takotsubo syndrome). Important diagnostic findings, management strategies and prognostic data for these non-atherosclerotic mechanisms of ACS are reviewed.
Key points
Non-atherosclerotic mechanisms contribute to a substantial number of acute coronary syndrome (ACS) events and require specific diagnostic and therapeutic strategies.
Spontaneous coronary artery dissection is most common in younger women, and the preferred strategy of conservative management carries an important risk of early clinical deterioration.
Coronary embolus can lead to ACS through direct, paradoxical or iatrogenic mechanisms.
Coronary vasospasm can occur at the epicardial or microvascular level, and invasive coronary vasomotion testing can improve diagnosis.
Myocardial bridging is often asymptomatic but can lead to ACS in an important subset of patients; invasive physiological testing might be necessary for diagnosis.
Stress-induced cardiomyopathy (Takotsubo syndrome) is frequently associated with endothelial dysfunction and can acutely cause outflow tract obstruction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arbustini, E. et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 82, 269–272 (1999).
Higuma, T. et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patient with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc. Interv. 8, 1166–1176 (2015).
Davies, M. J. et al. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N. Engl. J. Med. 310, 1137–1140 (1984).
Hochman, J. S. et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N. Engl. J. Med. 341, 226–232 (1999).
Gehrie, E. R. et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines Quality Improvement Initiative. Am. Heart J. 158, 688–694 (2009).
Chokshi, N. P. et al. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patient with acute coronary syndromes. Clin. Cardiol. 33, 495–501 (2010).
Roe, M. T. et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. The platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy trial investigators. Circulation 102, 1101–1106 (2000).
Emond, M. et al. Long-term survival of medically treat patients in the Coronary Artery Surgery Study (CASS) registry. Circulation 90, (2645–2657 (1994).
Patel, M. R. et al. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 362, 886–895 (2010).
Tweet, M. S. et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 126, 579–588 (2012).
Hayes, S. N. et al. Spontaneous coronary artery dissection: current state of the science: a Scientific Statement from the American Heart Association. Circulation 137, e523–e557 (2018).
Tweet, M. S. et al. Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. Mayo Clin. Proc. 86, 845–850 (2011).
Mortensen, K. H. et al. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter. Cardiovasc. Interv. 74, 710–717 (2009).
Vanzetto, G. et al. Prevalence, therapeutic management, and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur. J. Cardiothorac. Surg. 35, 250–254 (2009).
Nishiguchi, T. et al. Prevalence of spontaneous coronary artery dissection in young patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 5, 263–270 (2016).
Nakashimia, T. et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int. J. Cardiol. 207, 341–348 (2016).
Rashid, H. N. et al. Incidence and characterization of spontaneous coronary artery dissection as a cause of acute coronary syndrome: a single-centre Australian experience. Int. J. Cardiol. 202, 336–338 (2016).
Saw, J. et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ. Cardiovasc. Interv. 7, 645–655 (2014).
Saw, J. et al. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter. Cardiovasc. Interv. 84, 1115–1122 (2014).
Fahmy, P. et al. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc. Interv. 9, 866–868 (2016).
Prasad, M. et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am. J. Cardiol. 115, 1672–1677 (2015).
Saw, J. et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc. Interv. 6, 44–52 (2013).
Rogowski, S. et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter. Cardiovasc. Interv. 89, 59–68 (2017).
Alfonso, F. et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc. Interv. 5, 1062–1070 (2012).
Tweet, M. S. et al. Spontaneous coronary artery dissection associated with pregnancy. J. Am. Coll. Cardiol. 70, 426–435 (2017).
Liang, J. J. et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J. Cardiovasc. Comput. Tomogr. 8, 189–197 (2014).
Schievink, W. I. Spontaneous dissection of the carotid and vertebral arteries. N. Engl. J. Med. 344, 898–906 (2001).
Volker, W. et al. The outer arterial wall layers are primarily affected in spontaneous cervical artery dissection. Neurology 76, 1463–1471 (2011).
Alfonso, F. et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J. Am. Coll. Cardiol. 59, 1073–1079 (2012).
Jackson, R. et al. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovasc. Imaging https://doi.org/10.1016/j.jcmg.2019.01.015 (2019).
Waterbury, T. M. et al. Early natural history of spontaneous coronary artery dissection. Circ. Cardiovasc. Interv. 11, e006772 (2018).
Kwon, T. G. et al. Proliferation of coronary adventitial vasa vasorum in patients with spontaneous coronary artery dissection. JACC Cardiovasc. Imaging 9, 891–892 (2016).
Waterbury, T. M. et al. Coronary endothelial function and spontaneous coronary artery dissection. Eur. Heart J. Acute Cardiovasc. Care https://doi.org/10.1177/2048872618795255 (2018).
White, S. K. et al. Prevalence of coronary vasospasm using coronary reactivity testing in patients with spontaneous coronary artery dissection. Am. J. Cardiol. 123, 1812–1815 (2019).
Eleid, M. F. et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ. Cardiovasc. Interv. 7, 656–662 (2014).
Eleid, M. F. et al. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography. Eur. Heart J. Acute Cardiovasc. Care 7, 609–613 (2017).
Tweet, M. S. et al. Spontaneous coronary artery dissection: acute findings on coronary computed tomography angiography. Eur. Heart J. Acute Cardiovasc. Care 8, 467–475 (2019).
Adlam, D. et al. European Society of Cardiology, Acute Cardiovascular Care Association, SCAD Study Group: a position paper on spontaneous coronary artery dissection. Eur. Heart J. 39, 3353–3368 (2018).
Tweet, M. S. et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ. Cardiovasc. Interv. 7, 777–786 (2014).
Lettieri, C. et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am. J. Cardiol. 116, 66–73 (2015).
Adlam, D. et al. Management of spontaneous coronary artery dissection in the primary percutaneous coronary intervention era. J. Invasive Cardiol. 22, 549–553 (2010).
Yumoto, K. et al. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc. Interv. 7, 817–819 (2014).
Alkhouli, M. et al. Coronary artery fenestration prior to stenting in spontaneous coronary artery dissection. Catheter. Cardiovasc. Interv. 88, e23–e27 (2016).
Shibata, T. et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation 132, 241–250 (2015).
Raphael, C. E. et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc. Interv. 11, 172–180 (2018).
Cheng, T. O. Coronary embolism. Int. J. Cardiol. 136, 1–3 (2009).
Brunson, J. G. et al. Coronary embolism in bacterial endocarditis. Am. J. Pathol. 29, 689–701 (1953).
Lacunza-Ruiz, F. J. et al. Coronary embolism and thrombosis of prosthetic mitral valve. JACC Cardiovasc. Interv. 7, e127–e128 (2014).
Matsuyama, T. A. et al. Critical multi-organ emboli originating from collapsed, vulnerable caseous mitral annular calcification. Pathol. Int. 62, 496–499 (2012).
El Sabbagh, A. et al. Cardiac myoxoma: the great mimicker. JACC Cardiovasc. Imaging 10, 203–206 (2017).
Garachemani, A. et al. Paradoxical emboli through the patent foramen ovale as the suspected cause of myocardial and renal infarction in a 48-year-old woman. Catheter. Cardiovasc. Interv. 70, 1010–1012 (2007).
Myers, P. O. et al. Impending paradoxical embolism. Chest 137, 164–170 (2010).
Rallidis, L. S. et al. Myocardial infarction under the age of 36: prevalence of thrombophilic disorders. Thromb. Haemost. 90, 272–278 (2003).
Dauvergne, C. et al. Acute myocardial infarction after left-heart catheterization in a patient with severe calcified bicuspid aortic stenosis. JACC Cardiovasc. Interv. 7, e5–e6 (2014).
Prizel, K. R., Hutchins, G. M. & Bulkley, B. H. Coronary artery embolism and myocardial infarction. Ann. Intern. Med. 51, 155–161 (1978).
Protasiewick, M., Rojek, A., Gajek, J. & Mysiak, A. Cardiac arrest due to left circumflex coronary artery embolism as a complication of subtherapeutic oral anticoagulation in a patient with mitral and aortic mechanical valve prostheses. Postepy Kardiol. Interwencyjnej 9, 97–100 (2013).
Boekholdt, S. M. et al. Arterial thrombosis and the role of thrombophilia. Semin. Thromb. Hemost. 33, 588–596 (2007).
Kaski, J. C. et al. Reappraisal of ischemic heart disease: fundamental role of coronary microvascular dysfunction in the pathogenesis of angina pectoris. Circulation 138, 1463–1480 (2018).
Oliva, P. B., Potts, D. E. & Pluss, R. G. Coronary arterial spasm in Prinzmetal angina — documentation by coronary arteriography. N. Engl. J. Med. 288, 745–751 (1973).
Picard, F. et al. Vasospastic angina: a literature review of current evidence. Arch. Cardiovasc. Dis. 112, 44–55 (2018).
Prinzmetal, M. et al. Angina pectoris: I. A variant form of angina pectoris: preliminary report. Am. J. Med. 27, 375–388 (1959).
Pristipino, C. et al. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation 101, 1102–1108 (2000).
Beltrame, J. F., Sasayama, S. & Maseri, A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J. Am. Coll. Cardiol. 33, 1442–1452 (1999).
Nakayama, N. et al. Clinical features and prognosis of patients with coronary spasm-induced non-ST-segment elevation acute coronary syndrome. J. Am. Heart Assoc. 3, e000795 (2014).
Ong, P. et al. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients with Acute Coronary Syndrome) study. J. Am. Coll. Cardiol. 52, 523–527 (2008).
JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 78, 2779–2801 (2014).
Hung, M. J., Hu, P. & Hung, M. Y. Coronary artery spasm: review and update. Int. J. Med. Sci. 11, 1161–1171 (2014).
Morita, S. et al. Differences and interactions between risk factors for coronary spasm and atherosclerosis — smoking, aging, inflammation, and blood pressure. Intern. Med. 53, 2663–2670 (2014).
Sugiishi, M. & Takatsu, F. Cigarette smoking is a major risk factor for coronary spasm. Circulation 87, 76–79 (1993).
Maseri, A. et al. Coronary artery spasm and vasoconstriction: the case for a distinction. Circulation 81, 1983–1991 (1990).
Yasue, H. et al. Coronary artery spasm — clinical features, diagnosis, pathogenesis, and treatment. J. Cardiol. 51, 2–17 (2008).
Kugiyama, K. et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation 94, 266–271 (1996).
Kugiyama, K. et al. Nitric oxide-mediated flow-dependent dilation is impaired in coronary arteries in patients with coronary spastic angina. J. Am. Coll. Cardiol. 30, 920–926 (1997).
Kandabashi, T. et al. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1β. Circulation 101, 1319–1323 (2000).
Shimokawa, H., Sunamura, S. & Satoh, K. RhoA/Rho-kinase in the cardiovascular system. Circ. Res. 118, 352–366 (2016).
Egashira, K. et al. Basal release of endothelium-derived nitric oxide at site of spasm in patients with variant angina. J. Am. Coll. Cardiol. 27, 1444–1449 (1996).
Miwa, K. et al. Increased oxidative stress with elevated serum thioredoxin level in patients with coronary spastic angina. Clin. Cardiol. 26, 177–181 (2003).
Shin, E. S. et al. Thrombus and plaque erosion characterized by optical coherence tomography in patients with vasospastic angina. Rev. Esp. Cardiol. 70, 459–466 (2017).
Ohyama, K. et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J. Am. Coll. Cardiol. 71, 414–425 (2018).
Murase, Y. et al. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur. Heart J. 25, 970–977 (2004).
Satake, K. et al. Relation between severity of magnesium deficiency and frequency of anginal attacks in men with variant angina. J. Am. Coll. Cardiol. 28, 897–902 (1996).
Miwa, K. et al. Alterations of autonomic nervous activity preceding nocturnal variant angina: sympathetic augmentation with parasympathetic impairment. Am. Heart J. 135, 762–771 (1998).
Burillo-Putze, G. et al. Incidence and impact of undisclosed cocaine use in emergency department chest pain and trauma patients. Int. J. Emerg. Med. 1, 169–172 (2008).
Lange, R. A. et al. Cocaine-induced coronary-artery vasoconstriction. N. Engl. J. Med. 321, 1557–1562 (1989).
McCord, J. et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 117, 1897–1907 (2008).
Moliterno, D. J. et al. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N. Engl. J. Med. 330, 454–459 (1994).
Wilbert-Lampen, U. et al. Cocaine increases the endothelial release of immunoreactive endothelin and its concentrations in human plasma and urine: reversal by coincubation with sigma-receptor antagonists. Circulation 98, 385–390 (1998).
Mo, W. et al. Role of nitric oxide in cocaine-induced acute hypertension. Am. J. Hypertens. 11, 708–714 (1998).
Shen, W.-K. et al. Sudden unexpected nontraumatic death in 54 young adults: a 30 year population-based study. Am. J. Cardiol. 76, 148–152 (1995).
Hollander, J. E. et al. Prospective multicenter evaluation of cocaine-associated chest pain. cocaine associated chest pain (COCHPA) study group. Acad. Emerg. Med. 1, 330–339 (1994).
Sohn, S. M. et al. Impact of alcohol drinking on acetylcholine-induced coronary artery spasm in Korean populations. Atherosclerosis 268, 163–169 (2018).
El Menyar, A. A. Drug-induced myocardial infarction secondary to coronary artery spasm in teenagers and young adults. J. Postgrad. Med. 52, 51–56 (2006).
Beltrame, J. F. et al. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 38, 2565–2568 (2017).
Araki, H. et al. Diurnal distribution of ST-segment elevation and related arrhythmias in patients with variant angina: a study by ambulatory ECG monitoring. Circulation 67, 995–1000 (1983).
Nakamura, M., Takeshita, A. & Nose, Y. Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina. Circulation 75, 1110–1116 (1987).
Feldman, R. L. et al. Electrocardiographic changes with coronary artery spasm. Am. Heart J. 106, 1288–1297 (1983).
Tanaka, A. et al. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J. Am. Coll. Cardiol. 58, 1608–1613 (2011).
Yamagishi, M. et al. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J. Am. Coll. Cardiol. 23, 352–357 (1994).
MacAlpin, R. N. Some observations on and controversies about coronary arterial spasm. Int. J. Cardiol. 181, 389–398 (2015).
Yasue, H. et al. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation 74, 955–963 (1986).
Okumura, K. et al. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J. Am. Coll. Cardiol. 12, 883–888 (1988).
Heupler, F. A. Jr et al. Ergonovine maleate provocative test for coronary arterial spasm. Am. J. Cardiol. 41, 631–640 (1978).
Takagi, Y. et al. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur. Heart J. 34, 258–267 (2013).
Inami, T. et al. Left ventricular dysfunction due to diffuse multiple vessel coronary artery spasm can be concealed in dilated cardiomyopathy. Eur. J. Heart Fail. 14, 1130–1138 (2012).
Cho, S. W. et al. Clinical outcomes of vasospastic angina patients presenting with acute coronary syndrome. J. Am. Heart Assoc. 5, e004336 (2016).
Hung, M. J. et al. Coronary vasospasm-induced acute coronary syndrome complicated by life-threatening cardiac arrhythmias in patients without hemodynamically significant coronary artery disease. Int. J. Cardiol. 117, 37–44 (2007).
Kundu, A. et al. Variant angina and aborted sudden cardiac death. Curr. Cardiol. Rep. 20, 26 (2018).
Ishii, M. et al. Acetylcholine-provoked coronary spasm at site of significant organic stenosis predicts poor prognosis in patients with coronary vasospastic angina. J. Am. Coll. Cardiol. 66, 1105–1115 (2015).
Task Force Members. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 34, 2949–3003 (2013).
Severi, S. et al. Long-term prognosis of “variant” angina with medical treatment. Am. J. Cardiol. 46, 226–232 (1980).
Nishigaki, K. et al. Prognostic effects of calcium channel blockers in patients with vasospastic angina — a meta-analysis. Circ. J. 74, 1943–1950 (2010).
Lombardi, M. et al. Efficacy of isosorbide-5-mononitrate versus nifedipine in preventing spontaneous and ergonovine-induced myocardial ischaemia: a double-blind, placebo-controlled study. Eur. Heart J. 14, 845–851 (1993).
Yasue, H. et al. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J. Am. Coll. Cardiol. 51, 1742–1748 (2008).
Masumoto, A. et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 105, 1545–1547 (2002).
Kishida, H. & Murao, S. Effect of a new coronary vasodilator, nicorandil, on variant angina pectoris. Clin. Pharmacol. Ther. 42, 166–174 (1987).
Teragawa, H. et al. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest 118, 1690–1695 (2000).
Miwa, K., Kambara, H. & Kawai, C. Effect of aspirin in large doses on attacks of variant angina. Am. Heart J. 105, 351–355 (1983).
Park, J. Y. et al. Impact of low-dose aspirin on coronary artery spasm as assessed by intracoronary acetylcholine provocation test in Korean patients. J. Cardiol. 60, 187–191 (2012).
Tanabe, Y. et al. Limited role of coronary angioplasty and stenting in coronary spastic angina with organic stenosis. J. Am. Coll. Cardiol. 39, 1120–1126 (2002).
Ciçek, D., Kalay, N. & Müderrisoglu, H. Incidence, clinical characteristics, and 4-year follow-up of patients with isolated myocardial bridge: a retrospective, single-center, epidemiologic, coronary arteriographic follow-up study in southern Turkey. Cardiovasc. Revasc. Med. 12, 25–28 (2011).
Möhlenkamp, S. et al. Update on myocardial bridging. Circulation 106, 2616–2622 (2002).
Tsujita, K. et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am. J. Cardiol. 103, 1344–1348 (2009).
Migliore, F. et al. LAD coronary artery myocardial bridging and apical ballooning syndrome. JACC Cardiovasc. Imaging 6, 32–41 (2013).
Migliore, F. et al. Myocardial bridging, apical ballooning syndrome and myocardial stunning: shall we connect the dots? Int. J. Cardiol. 168, 3109–3111 (2013).
Zorzi, A. et al. Electrocardiographic J waves as a hyperacute sign of Takotsubo syndrome. J. Electrocardiol. 45, 353–356 (2012).
Zorzi, A. et al. Relationship between repolarization abnormalities and myocardial edema in atypical Tako-Tsubo syndrome. J. Electrocardiol. 46, 348–351 (2013).
Ishikawa, Y. et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation 120, 376–383 (2009).
Kim, J. W. et al. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am. J. Cardiol. 100, 1083–1086 (2007).
Corban, M. T. et al. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J. Am. Coll. Cardiol. 63, 2346–2355 (2014).
Tarantini, G. et al. Unmasking myocardial bridge-related ischemia by intracoronary functional evaluation. Circ. Cardiovasc. Interv. 11, e006247 (2018).
Tarantini, G. et al. Left anterior descending artery myocardial bridging: a clinical approach. J. Am. Coll. Cardiol. 68, 2887–2899 (2016).
Angelini, P., Trivellato, M., Donis, J. & Leachman, R. D. Myocardial bridges: a review. Prog. Cardiovasc. Dis. 26, 75–88 (1983).
Escaned, J. et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J. Am. Coll. Cardiol. 42, 226–233 (2003).
Davies, J. E. et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N. Engl. J. Med. 376, 1824–1834 (2017).
Götberg, M. et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 376, 1813–1823 (2017).
Konen, E. et al. The prevalence and anatomical patterns of intramuscular coronary arteries: a coronary computed tomography angiographic study. J. Am. Coll. Cardiol. 49, 587–593 (2007).
Koo, H. J. et al. CT-based myocardial ischemia evaluation: quantitative angiography, transluminal attenuation gradient, myocardial perfusion, and CT-derived fractional flow reserve. Int. J. Cardiovasc. Imaging 32 (Suppl. 1), 1–19 (2016).
Yoon, Y. E. et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc. Imaging 5, 1088–1096 (2012).
Sato, H., Tateishi, H. & Uchida, T. in Clinical Aspect of Myocardial Injury: Ischemia to Heart Failure Ch. 4 (eds Kodama, K., Haze, K. & Hori, M.) 56-64 (Kagakuhyoronsha Publishing Co, 1990).
Pavin, D., Le Breton, H. & Daubert, C. Human stress cardiomyopathy mimicking acute myocardial syndrome. Heart 78, 509–511 (1997).
Sharkey, S. W., Shear, W., Hodges, M. & Herzog, C. A. Reversible myocardial contraction abnormalities in patients with an acute noncardiac illness. Chest 114, 98–105 (1998).
Desmet, W. J., Adriaenssens, B. F. & Dens, J. A. Apical ballooning of the left ventricle: first series in white patients. Heart 89, 1027–1031 (2003).
Wittstein, I. S. et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 352, 539–548 (2005).
Lyon, A. R. et al. Current state of knowledge on Takotsubo syndrome: a position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 18, 8–27 (2016).
Pelliccia, F. et al. Takotsubo is not a cardiomyopathy. Int. J. Cardiol. 254, 250–253 (2018).
Templin, C. et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 373, 929–938 (2015).
Schneider, B. et al. Gender differences in the manifestation of Tako-Tsubo cardiomyopathy. Int. J. Cardiol. 166, 584–588 (2013).
Redfors, B. et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction — a report from the SWEDEHEART registry. Int. J. Cardiol. 185, 282–289 (2015).
Deshmukh, A. et al. Prevalence of takotsubo cardiomyopathy in the united states. Am. Heart J. 164, 66–71.e1 (2012).
Prasad, A., Lerman, A. & Rihal, C. S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am. Heart J. 155, 408–417 (2008).
Bybee, K. A. et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann. Intern. Med. 141, 858–865 (2004).
Akashi, Y. J., Nef, H. M. & Lyon, A. R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 12, 387–397 (2015).
Ghadri, J. R. et al. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur. Heart J. 37, 2823–2829 (2016).
Gianni, M. et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur. Heart J. 27, 1523–1529 (2006).
Kosuge, M. & Kimura, K. Electrocardiographic findings of takotsubo cardiomyopathy as compared with those of anterior acute myocardial infarction. J. Electrocardiol. 47, 684–689 (2014).
Frangieh, A. H. et al. ECG criteria to differentiate between Takotsubo (stress) cardiomyopathy and myocardial infarction. J. Am. Heart Assoc. 5, e003418 (2016).
Kurisu, S. et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ. J. 68, 77–81 (2004).
Kato, K., Lyon, A. R., Ghadri, J.-R. & Templin, C. Takotsubo syndrome: aetiology, presentation and treatment. Heart 103, 1461–1469 (2017).
Pelliccia, F., Kaski, J. C., Crea, F. & Camici, P. G. Pathophysiology of takotsubo syndrome. Circulation 135, 2426–2441 (2017).
Naegele, M. et al. Endothelial function and sympathetic nervous system activity in patients with takotsubo syndrome. Int. J. Cardiol. 224, 226–230 (2016).
Camici, P. G. & Crea, F. Microvascular angina: a women’s affair? Circ. Cardiovasc. Imaging 8, e003252 (2015).
Ono, R. & Falcao, L. M. Takotsubo cardiomyopathy systematic review: pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int. J. Cardiol. 209, 196–205 (2016).
Citro, R. et al. Clinical profile and in-hospital outcome of Caucasian patients with Takotsubo syndrome and right ventricular involvement. Int. J. Cardiol. 219, 455–461 (2016).
Nguyen, T. H. et al. N-terminal pro-brain natriuretic protein levels in Takotsubo cardiomyopathy. Am. J. Cardiol. 108, 1316–1321 (2011).
Song, B. G. et al. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reserve or inverted Takotsubo cardiomyopathy: comparison with mid or apical variant. Clin. Cardiol. 34, 693–699 (2011).
Frohlich, G. M. et al. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int. J. Cardiol. 154, 328–332 (2012).
Izumo, M. & Akashi, Y. J. Role of echocardiography for takotsubo cardiomyopathy: clinical and prognostic implications. Cardiovasc. Diagn. Ther. 8, 90–100 (2018).
De Backer, O. et al. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in takotsubo cardiomyopathy: a two-year, two-center experience. BMC Cardiovasc. Disord. 14, 147 (2014).
Warisawa, T., Naganuma, T. & Nakamura, S. Reversible microvascular dysfunction in takotsubo syndrome shown using index of microcirculatory resistance. Circ. J. 80, 750–752 (2016).
Tornvall, P., Collste, O., Ehrenborg, E. & Jarnbert-Petterson, H. A case-control study of risk markers and mortality in takotsubo stress cardiomyopathy. J. Am. Coll. Cardiol. 67, 1931–1936 (2016).
Stiermaier, T. et al. Prevalence and clinical significance of life-threatening arrhythmias in Takotsubo cardiomyopathy. J. Am. Coll. Cardiol. 65, 2148–2150 (2015).
Icli, A. et al. Short-term warfarin treatment for apical thrombus in a patient with Takotsubo cardiomyopathy. Cardiovasc. J. Afr. 27, 1–3 (2016).
Bharathi, K. S., Kulkarni, S., Sadananda, K. S. & Gurudatt, C. L. Takotsubo cardiomyopathy precipitated by negative pressure pulmonary oedema following total thyroidectomy. Indian J. Anaesth. 60, 202–205 (2016).
Lu, D. Y. et al. Ventricular septal defect from takotsubo syndrome. Case Rep. Cardiol. 2016, 2693062 (2016).
Jaguszewski, M. et al. Ventricular rupture in Takotsubo cardiomyopathy. Eur. Heart J. 33, 1027 (2012).
Sharkey, S. W. et al. Natural history and expansive clinical profile of stress (Tako-Tsubo) cardiomyopathy. J. Am. Coll. Cardiol. 55, 333–341 (2010).
Song, B. G. et al. The impact of stressor patterns on clinical features in patients with Tako-Tsubo cardiomyopathy: experiences of two tertiary cardiovascular centers. Clin. Cardiol. 35, E6–E13 (2012).
Roffi, M. et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 37, 267–315 (2016).
Steg, H. et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force on the management of acute ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur. Heart J. 33, 2569–2619 (2012).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 64, e139–e228 (2014).
O’Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013).
Isogai, T., Matsui, H., Tanaka, H., Fushimi, K. & Yasunaga, H. Early β-blocker use and in-hospital mortality in patients with Takotsubo cardiomyopathy. Heart 102, 1029–1035 (2016).
Migliore, F. et al. Incidence and management of life-threatening arrhythmias in Takotsubo syndrome. Int. J. Cardiol. 166, 261–263 (2013).
Bietry, R., Reyentovich, A. & Katz, S. D. Clinical management of Takotsubo cardiomyopathy. Heart Fail. Clin. 9, 177–186 (2013).
Singh, K. et al. Systematic review and meta-analysis of incidence and correlates of recurrence of Takotsubo cardiomyopathy. Int. J. Cardiol. 174, 696–701 (2014).
Author information
Authors and Affiliations
Contributions
T.M.W. researched the data for the article, and all the authors wrote the manuscript. T.M.W., B.J.G. and R.G. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Waterbury, T.M., Tarantini, G., Vogel, B. et al. Non-atherosclerotic causes of acute coronary syndromes. Nat Rev Cardiol 17, 229–241 (2020). https://doi.org/10.1038/s41569-019-0273-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0273-3
This article is cited by
-
Neutrophil-to-lymphocyte ratio as a predictor of in-hospital complications and overall mortality in Takotsubo syndrome preceded by physical triggers
BMC Cardiovascular Disorders (2023)
-
Acute myeloid leukemia causing acute thrombosis of the coronary arteries: a case report
Journal of Medical Case Reports (2022)
-
Nomogram to predict recurrent chest pain in patients with myocardial bridging
European Radiology (2022)
-
Association between age and infection in patients with acute ST-elevation myocardial infarction
The Egyptian Heart Journal (2021)
-
Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease
Cardiovascular Intervention and Therapeutics (2021)