Abstract
Since their initial presentation in 2005 by Yaghi and co-workers, covalent organic frameworks (COFs) have been the focus of much development. Here we introduce mechanical bonds into COFs by employing a method that involves supramolecular self-assembly, dynamic imine-bond formation, and liquid-liquid interfacial condensation and produces a continuous 2D polyrotaxaneted film. A diamino-viologen linker is complexed with the macrocycle cucurbit[7]uril (CB[7]) to form pseudorotaxanes that then condense with an aromatic tri-aldehyde core at the interface of two solvents. The unrotaxanated COF is prepared by condensing the viologen and the trialdehyde in the absence of CB[7], and the chemical and physical properties of the formed films are compared. The CB[7]–encapsulated film is mechanically and thermally more robust and more luminescent. The synthetic strategy presented here allows the incorporation of mechanically interlocked molecules (MIMs) into polymeric materials and provides access to materials that have properties and functionalities that are yet to be explored.
Similar content being viewed by others
Introduction
The immobilization of mechanically interlocked molecules (MIMs)1,2,3, such as rotaxanes3,4,5,6, catananes7,8, or knots9,10 into polymeric materials, became an appealing research area in view of all the effects that the mechanical bond has on the chemical and physical properties of materials. Ionic covalent organic frameworks (ICOFs)11,12,13,14,15,16,17 are potentially useful as ion exchangers13 and conductors12, solid catalysts13, and solid electrolytes12, and as such have been the focus of much recent attention in materials chemistry. The presence of ionic building blocks in ICOFs18 gives rise to novel properties that are distinct from those associated with covalent organic frameworks (COFs) composed of neutral components19. Moreover, charged components in ICOFs might serve as high-affinity recognition stations for shape- and charge-complementary macrocycles and thereby offer an opportunity for greater structural diversity20.
Owing to their rich redox chemistry18 and their ability to form inclusion complexes with a number of macrocyclic receptors such as crown ethers21, calixarenes22, cyclodextrins23, cucurbiturils24,25, viologens, i.e., 4,4′-bipyridinium ions, are among the most studied ionic moieties in supramolecular chemistry26,27,28. To date, several viologen-based ICOFs have been synthesized by conventional methods15,18,29,30; however, the products of these usually high-temperature reactions tend to be insoluble, microcrystalline powders with morphologies that are difficult to control and that have a limited range of practical application. Interfacial polymerization is an alternative strategy for the fabrication of thin membranes of amorphous polymers31, and more recently 2D COFs17,32,33. Because this method can be carried out at room temperature, it does not significantly hinder supramolecular interactions, and therefore allows for the use of thermally labile complexes such as pseudorotaxanes and their incorporation in the form of MIMs into covalently bonded polymeric networks.
Cucurbiturils (CB[n]s, n = 5, 6, 7, 8, or 10) are a family of glycouril-based macrocycles34 that have two identical carbonyl-rimmed portals, a hydrophobic cavity that can hold neutral or cationic guest molecules, and an overall pumpkin-like shape. CB[n]s have often been used as components for the self-assembly of mechanically interlocked molecules35 and molecular switches36,37,38. Furthermore, CB[7], in particular, has drawn attention because of its increased solubility in water compared to its congeners24. Moreover, it forms stable inclusion complexes with viologen derivatives39. Extended networks that incorporate rotaxane-like subunits are dynamic systems in which the macrocyclic components retain some freedom of movement while the frameworks they encircle remain fixed. The macrocycles insulate and stabilize the organic struts and can provide greater thermal stability and enhance the mechanical properties of the overall structure40. Mechanically interlocked molecules such as [2]rotaxanes were successfully incorporated into metal-organic frameworks41,42,43 and coordination polymers44 but yet to be in purely organic 2D or 3D extended materials.
Here we describe the threading of amine-functionalized organic struts containing viologen through CB[7] to form a polyrotaxanated organic network that has enhanced mechanical and photophysical properties, as compared to the corresponding unrotaxanated framework.
Results
Synthesis and characterization
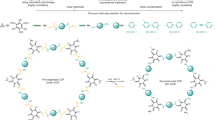
First, we synthesized a viologen-based COF (denoted TpV, Fig. 1a) via interfacial liquid–liquid polymerization (Fig. 1b). An aqueous solution of 1,1′-bis(4-aminophenyl)-[4,4′-bipyridine]-1,1′-diium dichloride (V•2Cl) and the catalyst p-toluene sulfonic acid (PTSA) was layered on top of an organic solution of 1,3,5-triformylphloroglucinol (Tp), the precursor to the core moiety of the framework. The resulting product of this reaction was a continuous self-standing thin film of a highly ordered cationic imine-linked COF whose lateral dimensions were dictated by the dimensions of the polymerization flask.
Synthesis of TpV and TpVCB[7]. a Chemical structures and photos of the free-standing films prepared by interfacial polymerization from the Schiff-base condensation of 1,1′-bis(4-aminophenyl)-[4,4′-bipyridine]-1,1′-diium dichloride (V•2Cl) and 1,3,5-triformylphloroglucinol (Tp) in the presence of catalytic amount of p-toluenesulfonic acid (PTSA). b The aqueous solution of the viologen linker and the catalyst in absence or presence of CB[7] was layered on top of the organic solution of the trialdehyde core, as shown in the schematic representation. c The corresponding polyrotaxanated material obtained under the same conditions but in the presence of CB[7]
Taking advantage of the water solubility of CB[7] and its ability to form inclusion complexes with viologen derivatives, we then synthesized the corresponding rotaxanated film (TpVCB[7]). In this synthesis, an equimolar aqueous solution of CB[7], our viologen diamine linker (V•2Cl), and catalytic amount of PTSA was layered on top of the organic solution of the Tp aldehyde core (Fig. 1c). The resultant thin film (TpVCB[7]) was amorphous and exhibited enhanced luminescent properties compared to TpV. The thickness of the obtained film was ~0.9 nm, which corresponds to the diameter of CB[7] and is consistent with incorporation of the macrocycle within the polymeric product45. Notably, increasing the ratio of CB[7] to two equivalents lead to the formation of a precipitate, which can be explained by the formation of a ternary 2:1 (CB[7])2:V2+ complex39,46 wherein two CB[7] macrocycles encircle the terminal amino-benzene moities of the viologen and hinder imine-bond formation39. FTIR, solid-state NMR, steady-state fluorescence and EPR spectroscopies provided further evidence for the presence of CB[7] within the film. As anticipated, the CB[7]-shielded network exhibited enhanced thermal, chemical, and mechanical stabilities compared to the CB[7]-free framework. These results demonstrate the usefulness of interfacial polymerization for incorporating mechanical bonds within 2D polymeric networks and support the hypothesis that host–guest chemistry can enhance the chemical and physical properties of the resulting films relative to the corresponding unrotaxanated ones. Computer simulations of the two systems were in good agreement with experimental observations. The changes in the structure upon CB[7] coordination were characterized in atomic detail and correlated with changes in mechanical properties. Based on the simulations we propose a possible mechanism for the enhanced mechanical strength observed after macrocycle complexation.
The TpV-COF was synthesized using liquid–liquid interfacial polymerization by layering an aqueous solution (10 mL) of the viologen-diamine monomer 1,1′-bis(4-aminophenyl)-[4,4′-bipyridine]-1,1′-diium dichloride (V•2Cl), (45.9 mg, 0.112 mmol) and the PTSA catalyst (38.5 mg, 0.224 mmol) over a 10 mL dichloromethane solution of 1,3,5-triformylphloroglucinol (Tp, 15.7 mg, 0.075 mmol). The above mentioned procedure was used to synthesize the CB[7]-encircled TpVCB[7] material by layering an aqueous solution (10 mL) of V•2Cl (20.5 mg, 0.05 mmol), CB[7] (58.1 mg, 0.05 mmol), and PTSA (38.5 mg, 0.224 mmol) over a dichloromethane solution (10 mL) of triformylphloroglucinol (Tp, 15.7 mg, 0.075 mmol).
Polymerization occurred at the interface of the two phases and provided continuous free-standing films that could be easily removed with tweezers after reaction completion. Stretching bands at 3186–3305 cm–1 (primary amine N–H) and 1639 cm–1 (aldehyde C=O) originally present in the spectra of the viologen and the glucinol monomers, respectively (Supplementary Fig. 1), are absent in the FTIR spectra of both unrotaxanated and rotaxanated materials, which indicates complete consumption of starting precursors during condensation. Moreover, the absence of alcohol O–H and imine C=N stretching bands in the spectra of the two materials and the appearance of a new peak at 1578 cm−1 that arises from C=C stretching clearly demonstrate that the structures are in the keto form. The stretching band of the C=O at 1616 cm−1 is merged with the C=C stretching band and appears as a shoulder. Analogous bands have been identified in the spectra of other imine-linked COFs that incorporate a 1,3,5-triformylphloroglucinol (Tp) core47. The FTIR spectrum of the TpVCB[7] film (Supplementary Fig. 1) displays an additional band at 1738 cm−1 that is consistent with the presence of the carbonyl groups of CB[7]. This peak is shifted relative to the C=O stretching band observed at 1714 cm−1 in the FTIR spectrum for CB[7], which indicates interaction between the macrocycles and viologen units of the TpVCB[7] framework. This shift is in agreement with literature data that characterize complexes in which CB[7] is threaded by viologen39,48.
For further characterization, we recorded 1H-13C cross-polarization/magic-angle spinning (CP-MAS) spectra of CB[7], TpV, and TpVCB[7] (Fig. 2). The broad peak at ~182 ppm in the spectra of both materials, TpV and TpVCB[7], corresponds to the presence of the keto groups. The spectra of both films also show a band that corresponds to exocyclic sp2-hybridized carbons (−C=C) at ∼107 ppm32. All other carbon resonances of the TpV COF are found between ~107 ppm and ~160 ppm. In the spectrum of TpVCB[7], the carbonyl peak of CB[7] appears at 157.1 ppm, while the peaks corresponding to CH and CH2 carbons show at 72.3 ppm and 54.0 ppm, respectively. These peaks are slightly shifted from their positions in the spectrum of CB[7] macrocycle. Both materials give rise to a peak at 20 ppm, which may be due to the –CH3 group of PTSA that is trapped within the structures. This signal has been identified in the spectra of other COFs whose syntheses were facilitated by PTSA catalysis49. We estimated the number of CB[7] units per viologen strut by employing solid-state direct polarization 13C MAS NMR spectroscopy (Supplementary Fig. 2). The quantitative spectra of TpVCB[7] were recorded with a recycle delay of 20 s, which is more than five times the longitudinal relaxation time (T1) of any of the carbons of CB[7] or the framework. Therefore, we were able to use the areas of the CH and CO carbon resonances to calculate the number of CB[7] macrocycles per viologen (SI for calculations). From peak areas in the 13C MAS spectra and knowing that each viologen unit has three CO carbons, whereas, each CB[7] unit has 14 CH carbons, we determined that there is approximately one CB[7] present per viologen.
Solid-state NMR analysis. Comparison of 13C CP-MAS spectra of CB[7], TpV, and TpVCB[7]. The C=O peak of the TpV framework (yellow shading) appears at ≈182 p.p.m, whereas the C=O peak of CB[7] (green shading) appears at 157.9 ppm. The –C=C peak of the framework (violet shading) and characteristic peaks for the –CH and CH2 carbons of CB[7] (gray and cyan shading, respectively) are also indicated
The structural features of both TpV and TpVCB[7] materials were characterized by low and wide angle PXRD analyses (Fig. 3). The low angle diffraction pattern for the TpV thin film, collected in transmission geometry, exhibits a sharp low angle diffraction signal (Fig. 3), confirming its ordered nature. This intense peak centered at 2θ = 2.1° is attributed to the (100) reflection of a hexagonal lattice. On the other hand, the wide angle PXRD pattern only shows broad features (Fig. S3), which is consistent with the absence of stacking order, as expected for the atomic thickness of the film. A crystal model consisting of honeycomb (hcb) layers formed by the linkage between the building blocks was built in the P-6 space group, and geometrically optimized with universal forcefield energy minimizations. The optimized crystal model unit cell parameters generated by simulated PXRD are a = b = 45.55 Å and c = 3.58 Å and are in agreement with those indicated by the experimental data (Fig. 3). The TpVCB[7] product does not show any low angle PXRD signal (Supplementary Fig. 3), which is attributed to the presence of bulky CB[7] macrocycles in the network, which probably distorts the layers’ order.
Structural characterization of TpV COF. a Comparison of the experimental low angle powder X-ray diffraction (PXRD) pattern (black) of the TpV thin film and the simulated PXRD peaks (red) of the eclipsed stacking model; b eclipsed space-filling model of the thin film in AA stacking mode; c Side view of a space-filling representation of two stacked layers. Tp (Orange), V•2Cl (blue), benzene (black), we omit the Cl for clarity
Nitrogen sorption measurements were carried out at 77 K to evaluate the surface areas and the porosities of the materials. The isotherms were classified as typical type II (Supplementary Fig. 4). The Brunauer−Emmett−Teller (BET) surface area and pore volume of the TpV film were found to be 106 m2 g–1 and 0.29 cm3 g−1, respectively. The relatively low porosity determined for TpV compared to neutral COFs was expected and can be attributed to pore blockage by chloride counter anions18. The CB[7]-threaded film has a lower N2 uptake capacity with a surface area of 20 m2 g–1 and a pore volume of 0.06 cm3 g−1, which reflects the presence of CB[7].
The morphology of both materials was characterized by field-emission scanning electron microscopy (FE-SEM), high-resolution transmission electron microscopy (HR-TEM), and atomic force microscopy (AFM). Scanning electron microscopy (SEM) images of the two membranes show sheet-like morphology (Fig. 4a, b). A key difference between the two films is that TpV shows a folded/curled structure compared to the rigid planar morphology of TpVCB[7]. High-resolution transmission electron microscopy (HR-TEM) images (Fig. 4c–f and Supplementary Figs. 5–6) of TpV and TpVCB[7] materials display 2D sheets, in agreement with the SEM studies. A clear Tyndall effect (Figs. S5a and S6a insets) was observed for aqueous solutions of each membrane, providing additional evidence for the presence of ultrathin sheets. The Tyndall effect could be observed for a long period of time, the solution remained stable even after 2 weeks (Supplementary Fig. 7). High magnification HRTEM images (Fig. 4e) of the TpV-COF exhibit lattice fringes with an average d-spacing value of 0.38 nm consistent with the corresponding PXRD analysis. No lattice fringes were observed in TpVCB[7]. Both films were further characterized by atomic force microscopy (AFM) (Fig. 4g, h and Supplementary Fig. 8), which also confirmed the presence of ultrathin sheet-like morphology. Height profile characterizations revealed different thicknesses of the two film types: 0.35 nm for TpV-COF versus 0.91 nm for TpVCB[7]. The 0.35 nm value for TpV is consistent with the layer d-spacing observed in the HR-TEM analysis (Fig. 4e inset) and indicates that the measured nanosheet is of single atom thickness. AFM topographic images of TpVCB[7] (Fig. 4h and Supplementary Fig. 9), show elliptical disc-like morphologies with raised edges/lips of 0.91 nm thickness, which corresponds to the height of the CB[7] cavity45.
Microscopic characterization. a, b SEM images, and c–f TEM images of TpV and TpVCB[7] films, respectively. Inset in e shows corresponding lattice fringes indicating crystallinity of TpV. g, h AFM images of TpV and TpVCB[7]. Scale bars = a 1 μm, b 50 μm, c 500 nm, d 500 nm, e 10 nm, f 200 nm, g 100 nm, and h 400 nm
Raman spectra (Supplementary Fig. 10) of the two films revealed D and G bands at 1366 cm–1 and 1587 cm–1, respectively, for TpV and at 1358 cm–1 and 1587 cm–1, respectively, for TpVCB[7]. Both spectra showed G-band-dominated structures. An overall decrease in intensity is observed with an increase in the intensity ratio of the D-band to the G-band, ID/IG, after the introduction of CB[7]. These results demonstrate that the membranes are graphene-like with the TpVCB[7]-based material being more disordered as a result of CB[7] complexation50,51,52.
Thermogravimetric analyses (TGA, Supplementary Fig. 11) show that both materials are thermally stable — to ~201 °C for TpV and to ~327 °C for TpVCB[7]. The greater stability of the TpVCB[7] film is a consequence of the encapsulation of the viologen struts by CB[7].
The chemical stability of the two films was evaluated by immersing them in boiling water, 3M HCl, or 3M NaOH solutions for 3 days. Upon PXRD analysis after 3 days of treatment, the relative peak intensities and positions in the spectrum of TpV remained mostly intact (Supplementary Figs. 12) with attenuation of the reflection peak at 2.1° for the material treated with 6M NaOH. FT-IR (Supplementary Fig. 13–14) and SEM (Supplementary Figs. 15–16) analyses of the treated films also showed no changes relative to the corresponding as-synthesized materials. The relatively enhanced resistance observed for TpV toward base can be explained by the fact that, at high pH, the viologen units are reduced to the radical cationic form as revealed by solid-sate EPR measurements of both films after base treatment (Supplementary Fig. 17c, d). The EPR intensity for TpVCB[7] is larger than the one observed for TpV indicating the extra stabilization of the viologen radical cation in TpVCB[7] as a result of CB[7] encircling the viologen struts.
The room temperature photoluminescent properties (Supplementary Fig. 18) of the two materials were studied by solid-state fluorescence spectroscopy. It is well known that viologen derivatives exhibit luminescence enhancement upon encapsulation in CB[7]39,53. Steady-state luminescence of TpV and TpVCB[7] in the solid-state were measured at room temperature. TpV displayed very weak luminescence with an emission maximum at λmax = 618 nm when excited at 375 nm. In contrast, TpVCB[7] showed a brilliant blue emission with λmax = 467 nm and eight-fold luminescence enhancement as compared to TpV, a clear sign of viologen's inclusion within the constituent CB[7] macrocycles.
Mechanical properties
SEM, TEM, and TGA studies suggested CB[7]-associated differences in membrane stiffness and stability that distinguished the two film types. In order to elucidate these differences and better understand the structure-mechanical property relationship of the materials, we investigated and compared the nanoscale mechanical properties of the two materials. We used two AFM-based techniques, namely (1) standard nano-indentation, which involves determining load-displacement (P–H) curves and (2) force versus distance (F/D) spectroscopy54,55,56, which measures the vertical force applied by the tip of a cantilever to a sample's surface during contact-AFM imaging.
Representative AFM images with marked locations used for F/D and nano-indentation data collection, as well as F/D and nano-indentation curves collected for TpV and TpVCB[7] films, respectively, are shown in Fig. 5. An adhesion energy of 50 ± 10 fJ and a “pull off force” of 2.7 ± 0.5 nN were obtained for the TpV film (Fig. 5c). The TpVCB[7] material exhibited a higher adhesion energy of 80 ± 10 fJ and a “pull of force” of 6.0 ± 0.5 nN (Fig. 5d). A higher adhesion energy, the sum of all energies of interaction, indicates a stronger affinity between the sample and the cantilever. Adhesion energy is directly proportional to the work of adhesion, which is defined as the specific energy needed to reversibly separate an interface into two free surfaces. When comparing two surfaces, the one with higher energy of adhesion and hence higher work of adhesion has the more stable surface. Our results indicate that the TpVCB[7] film is mechanically stronger than TpV.
AFM mechanical characterization of the films. a, b Preliminary AFM imaging: c, d typical F/D curve measured for one of the five different locations; e, f AFM nano-indentation data collected for TpV and TpVCB[7], respectively; g, h Bar diagram represent the value of Young Modulus and mechanical stiffness of TpV and TpVCB[7], respectively. Scale bars = a 300 nm and b 3 µm. The error bars g, h represent the standard deviation of three experiments
Representative load-displacement (P–H) plots for TpV and TpVCB[7] are shown in Fig. 5e–f, respectively. Young’s modulus values measured from the unloading curves for TpV and TpVCB[7] were found to be 1.9 ± 0.3 GPa and 3.0 ± 0.3 GPa, respectively (Fig. 5g), indicating higher mechanical stiffness for TpVCB[7]. Hardness (H) values were also obtained using H = Pmax/Acontact where Pmax is the maximum load and Acontact is the contact area established at maximum load. The hardness values were found to be 1.6 ± 0.5 MPa and 2.6 ± 0.5 MPa for TpV and TpVCB[7], respectively (Fig. 5h), which is consistent with the modulus values for the two films. It is also interesting to note that the loading curve for TpVCB[7] showed multiple “pop-outs”, which are probably indicative of multilayer stacks. We did not observe any “pop-outs” during analysis of TpV. The enhanced mechanical properties of the TpVCB[7] material might be due to its inherent stacking tendencies, which are analogous to nacre systems57. All of our mechanical studies indicate that threading the 2D viologen framework through CB[7] leads to the formation of a film that is 1.5 times stiffer and stable.
Computer simulations
To shed light on the structural and mechanical changes observed upon CB[7] complexation of the viologen framework, we used all-atom molecular dynamics (MD) simulation (Supplementary Note 1). Equilibrium conformations of TpV and TpVCB[7] are shown in Fig. 6 for comparison.
Computational study. Computer simulation model of a, b TpV and c, d TpVCB[7] structures. Two parallel layers (colored blue and pink) are used in molecular dynamics simulation. The Cl− counter ions were omitted for clarity. In the TpVCB[7] structure, the CB[7] macrocycles are colored red and orange and encircle the blue layer and the pink layer, respectively. It is worth noting that the simulated structure of TpVCB[7] does not reflect the real structure, which is amorphous. CB[7] (Red, Orange),TpV (Blue, Pink)
TpV show a minimal change from the X-ray structure model, while TpVCB[7] showed distorted π–π stacking interactions in addition to layer slippage with larger interlayer distance of 7.5 ± 0.4 nm compared to the, 3.6 ± 0.3 nm value computed for TpV. To better asses the intermolecular interactions governing the two systems, we computed the Young’s modulus, E, for each bilayer and compared it to the experimentally determined value (Supplementary Fig. 19). The computed E value for TpV, 2.0 ± 0.4 GPa, was found to be in remarkable agreement with the experimental value of 1.9 ± 0.3 GPa. The E value for TpVCB[7] calculated to be 5.0 ± 0.9 GPa, is comparable to the experimentally measured value of 3.0 ± 0.3 GPa. In short, both computed and measured Young’s moduli indicate that TpVCB[7] is mechanically more stable. Also, the good agreement between our computed and measured values increased our confidence in the validity of our computational model. To gain more insight into the greater mechanical stability of TpVCB[7], we computed the intermolecular energy for each bilayer. The sum of intermolecular interactions for the TpVCB[7] bilayer was found to be lower, which indicates greater stability. Interestingly, the major source of mechanical stabilization in the TpV bilayer appears to be the electrostatic forces between its positively charged linkers and the tightly bound chloride ions (Supplementary Table 1). In contrast, in TpVCB[7], the chloride ions are diffusely bound due to displacement by CB[7] macrocycles (Supplementary Fig. 20). CB[7] was found to have favorable intermolecular interactions with the rest of the membrane (Supplementary Fig. 21a). This is despite the macrocycle’s net charge being zero. The unique charge distribution of CB[7] creates a dipole moment that is orthogonal to the ring’s surface (Supplementary Fig. 21b). We found the magnitude of the dipole moment to be 8.30 Debye from ab initio methods and 5.80 Debye from the forcefield charges, both values being high enough to account for the intermolecular energy terms reported in (Supplementary Fig. 21a). In short, simulations suggests, favorable charge-dipole and dipole–dipole interactions leading to enhanced stability of the TpVCB[7] film.
Discussion
We have synthesized a free-standing viologen-linked covalent organic framework (TpV) by one-pot liquid–liquid interfacial polymerization. Using the same protocol, we fabricated a polyrotaxanated network (TpVCB[7]). Both constructs form flexible ultrathin films that are stable to hydrolysis in basic, neutral, or acidic aqueous solutions. AFM characterization showed that TpV material exhibits a mono layer thick (~0.38 nm) sheet-like morphology while elliptical disc-like morphologies with raised edges/lips with ~0.91 nm thickness were observed for TpVCB[7]. The mechanical properties of the materialss measured by AFM nano-indentation and F/D spectroscopy demonstrated that TpVCB[7] is stiffer than TpV. MD simulations confirm the higher stability of TpVCB[7] and provide an explanation based on molecular interactions. Furthermore, the TpVCB[7] product exhibited enhanced luminescence. We are currently applying host–guest chemistry and the interfacial synthetic method to develop other polyrotaxanated networks that have enhanced physical and photophysical properties. Our results represent a significant progress towards developing extended organic materials with mechanically interlocked molecules and all the advantages and additional functionalities they offer. Moreover, the developed approach opens the door to industrialization of MIM-containing materials and their use on a large scale.
Methods
Materials
All reagents and starting materials were purchased from Sigma–Aldrich and used without further purification. The precursor, 1,1'-bis(4-aminophenyl)-[4,4'-bipyridine]-1,1'-diium dichloride (V•2Cl), was synthesized according to the published procedure with no modifications58. Triformylphloroglucinol (Tp) was prepared from Phloroglucinol using literature procedure47. Deionized water was used from Millipore Gradient Milli-Q water purification system. Thin-layer chromatography (TLC) was performed on silica gel 60 F254 (E. Merck). The plates were inspected under UV light. Column chromatography was performed on silica gel 60F (Merck 9385, 0.040–0.063 mm). Routine nuclear magnetic resonance (NMR) spectra were recorded at 25 °C on a Bruker Avance spectrometer, with a working frequency of 500 MHz for 1H, and 75 MHz for 13C nuclei. All chemical shifts are reported in ppm relative to the signals corresponding to the residual non-deuterated solvents (CD3CN: δ = 1.94 ppm, CD3OD: δ = 3.31 ppm, D2O: δ = 4.97 ppm, and DMSO-d6: δ = 2.50 ppm). Coupling constant values (J) are given in hertz (Hz), the multiplicity is abbreviated in the following way: s (singlet) and d (doublet). Fourier transform infrared (FTIR) studies were carried out on the Agilent 670-IR spectrometer. Thermogravimetric analysis (TGA) was performed on TA SDT Q600. Scanning electron microscopy (SEM) images were obtained from FEI Quanta 450FEG. The topography of the self-templated samples was analyzed by dynamic atomic force microscopy (5500 Atomic Force Microscope; Keysight Technologies Inc., Santa Rosa, CA). We acquired topography, phase and amplitude scans simultaneously. Silicon cantilevers (NanosensorsTM, Neuchatel, Switzerland) with resonant frequencies of 250–300 kHz and force constants of 100–130 Nm─1 were used. The set point value was kept at 2.5 V. AFM scans were collected at 1024 points/lines with scan speed of 0.20 at fixed scan angle of 0o. Scan artifacts were minimized by acquiring a typical scan at an angle of 90o under identical image acquisition parameters. We used GwyddionTM free soſtware (version 2.47), SPM data visualization, and analysis tool for post-processing the AFM scans. Size and morphology of the Films were determined with a TEM (FEI-Titan 300) microscope. Samples were prepared on a carbon-coated copper grid. A drop of dispersed polymeric network was spotted on the grid and allowed to dry overnight. Surface area measurements were conducted on a Micromeritics 3Flex gas sorption analyzer. Samples (20–50 mg) were degassed at 85 °C for 24 h and then backfilled with N2. Adsorption isotherms were generated by incremental exposure to ultrahigh-purity nitrogen up to 1 atm in a liquid nitrogen bath, and surface parameters were determined using BET adsorption models included in the instrument software (Micromeritics ASAP 2020 V4.00). Low angle Powder X-ray diffraction (PXRD) data were collected with a Bruker diffractometer, equipped with a Cu INCOATED microsource, operated at 50 W power (50 kV, 1.00 mA) to generate Cu Kα radiation (λ = 1.54184 Å) and a Bruker VANTEC 500 area detector (MICROGAP technology). The films were placed inside borosilicate glass capillaries of 0.2 mm diameter and 0.01 mm wall thickness, and diffraction data were recorded by exposing the capillaries to the X-ray beam for 4 h period, with a sample to detector distance of 180 mm. The diffraction images were recorded and integrated with the APEX3 software package (Bruker. APEX3 v2015.5–2. Bruker AXS Inc., Madison, Wisconsin, U.S.A. 2015). Wide angle Powder X-ray diffraction (PXRD) measurements were carried out using the PANalytical X’Pert PRO MP X-ray diffractometer consisting of a focusing elliptical mirror and a fast high-resolution detector (PIXCEL) with the radiation wavelength of 0.15418 nm.
The solid-state steady-state and time-resolved fluorescence measurements of membranes solid-state were carried out using a solid sample holder, a LifeSpec II spectrometer that is based on the TCSPC method, excitation at 375 nm using an Edinburgh diode laser with a repetition rate of 20 MHz, a time resolution of 30 ps, and a red-sensitive high-speed PMT detector (Hamamatsu, H5773–04).
All solid-state NMR experiments were carried out on a Bruker Avance-HD 600 MHz spectrometer operating at a static field of 14.1 T, resonating at 150.0 MHz for 13C, using a 3.2 mm triple resonance MAS probe. Samples in the form of a fine powder were packed into a 3.2 mm O.D. zirconia rotor and spun at 24 kHz. 1H–13C CP/MAS measurements were performed using a standard Ramped-Amplitude Cross-Polarization (RAMPCP) pulse sequence59. The CP-MAS experiments were carried out using a recycle delay of 5 s and a contact time of 2.0 ms. The 13C direct excitation MAS experiments were carried out using a recycle delay of 20 s. 13C chemical shifts were referenced to the CH2 resonance observed for adamantane at 38.48 ppm with respect to the signal for neat TMS60. Raman measurements were performed with a Witec Alpha 300 confocal Raman system. The samples were illuminated with a 532 nm laser and an acquisition three times between 25 and 80 s.
Nano-mechanical testing of TpV and TpV CB[7] samples
Atomic force microscopy (Park NX10, Park Systems Korea) was used in true non-contact mode for morphological characterization. This unique scan mode prevents potentially invasive tip-sample interaction during a scan. AFM scans were collected using Park SmartScanTM software (Park Systems Korea) using ultra-sensitive (Super Sharp Standard NCH cantilevers, High Density Carbon tip, Manufacturer: NanoWorld AG, Switzerland) with a typical tip height of 10–15 µm and 2 nm tip radius, under ambient conditions. The physical dimensions of these cantilevers were 125 μm (length), 30 μm (width), and 4 μm (thickness), respectively. The force constant value and resonant frequency were 42 Nm─1 and 320 kHz, respectively. Images were recorded with a resolution of 1024 lines/image × 1024 points/line.
Mechanical properties
In addition to conventional AFM imaging, Park AFM has in built capabilities to precisely measure the nanoscale mechanical properties of materials, namely, nano-indentation technique (load-displacement (P–H) curve) and an additional capability referred to as the Force vs Distance (F/D) Spectroscopy. F/D spectroscopy measures the vertical force applied by the tip to the surface during contact-AFM imaging, through the deflection of the cantilever as a function of the extension of the piezoelectric scanner. Typically, this technique employs a significantly smaller force than that used in conventional AFM nano-indentation experiments. In F/D spectroscopy, the dependence of the cantilever deflection on the extension of the piezoelectric scanner is directly correlated to the tip-sample interaction forces reflecting a surface mechanical property. In principle, the technique could be used measure local variations in the elastic properties of the surface.
In our experiments, we typically collected AFM topographic scans of the Films before conducting F/D spectroscopic measurements. Our experimental procedure involved precisely marking a specific location on a Film sheet and collecting the F/D data from marked location. For each Film type, we obtained F/D data from five individual Film sheet per sample, and performed statistical analysis of these data sets.
We followed the above-mentioned procedure for AFM indentation also. In these experiments, AFM tip was moved downwards towards the sample marked location, at a constant loading rate of 30 µm•s─1 and the cantilever deflection versus probe displacement is acquired. Once the maximum load of 550 nN is reached, the tip was retracted at the same rate the unloading curve was acquired. The above data were used to generate the standard load-displacement (P–H) curves for TpV and TpVCB[7] Film samples, respectively. Poisson ratios of 0.07 (AFM tip) and 0.5 (TpV/TpCB[7] Film samples) were used. P–H curves were used to determine the Young’s modulus (E) and hardness (H) using the standard Oliver and Pharr method61.
Computer simulations
We employed all-atom Molecular dynamics simulation (MD) to study the structure and mechanical stability of TpV and TpVCB[7]. Constructing the initial models based on the crystal structure of TpV forming a bilayer, we created a periodic structure of each Film. We neutralized the simulation box by adding 12 Cl─ ions to each simulation box. All simulations are performed using LAMMPS62 suit of programs with forcefields generated using MAPS platform of Scienomics (Materials and Processes Simulations Platform, Version 4.2, Scienomics SARL, Paris, France). To represent molecular interactions we used Driedening-X6 Force Field63. First, we geometry optimized the structures by allowing volume and shape changes during 5000 steps of minimization using Conjugate Gradient method. The lowest energy structure was then used as starting point for equilibrium MD simulations where we freeze the polymer and relax Cl─ ions in canonical ensemble (NVT) for about 1 ns. Equations of motion were integrated using a velocity-Verlet (9) algorithm with a time step of 1fs at a temperature of T = 300K. Electrostatic interactions were computed using Particle Mesh Ewald summation algorithm64 with a distance cutoff of 12 Å and a relative force accuracy of 1.4 × 10−5 kcal/(mol Å). Lennard-Jones interactions were cutoff at the same distance with a tail correction added for more accurate representation of the long-range dispersion interactions. Last frame of the equilibration run was taken as the starting point for the production runs. Unrestrained simulations were employed by setting pressure to 1.0 bar and temperature to 300K using Nose-Hoover thermostat and barostat65,66, respectively. By allowing simulation box dimensions to change we monitor the unit cell dimensions, interlayer distance, and interaction energies from 100ns long MD simulations for the two systems studied, trajectory was recorded for every 1 ps for analysis.
To quantify the mechanical response, we computed the Young’s modulus, E by pulling the layers in the axis orthogonal to the interlayer plane forming the film. We estimated E from the slope of the initial linear part of the instantaneous stress/strain curve. For that purpose, the pressure on the perpendicular axis was varied with a constant rate from 0 atm to −1000 atm. To estimate the uncertainty in computations we repeated the simulations eight times using different conformations from the equilibrium simulations for each model. We report the mean and standard error based on these independent measurements. To assess the dependence of the result on the pulling rate we investigated E for the pulling rates range in two orders of magnitude (1–0.01 ps/atm) for TpV model. A relatively small dependence on the computed E on the pulling rate allows using the slowest pulling rate (r = 0.01 ps/atm) as the rate for also the TpVCB[7] model.
Synthesis of 1,1'-bis(4-aminophenyl)-[4,4'-bipyridine]-1,1'-diium dichloride
The title compound was prepared according to literature procedure with slight modification. (1) A mixture of 1,1'-bis(2,4-dinitrophenyl)-[4,4'-bipyridine]-1,1'-diium dichloride (0.5 g, 1.6 mmol) and three equivalents p-phenylenediamine (0.290 g, 4.8 mmol) were refluxed in 250 mL ethanol for 24 h. After the reaction completion, the reaction mixture was concentrated into 100 mL under reduced pressure and poured into 750 mL of THF. The precipitate was collected by filtration and washed with THF. The solid was redissolved in a minimum amount of ethanol with heating and precipitated in THF. The solid was filtered and dried in a vacuum-oven overnight at 40 °C to give the 1,1'-bis(4-aminophenyl)-[4,4'-bipyridine]-1,1'-diium dichloride in 72% yield. 1H NMR (500MHz, D2O-d4, 25 °C): δ 6.97 (d, 4H, J = 8.9 Hz, Ar-H), 7.52 (d, 4H, J = 8.9 Hz, Ar-H), 8.61 (d, 4H, J = 7.1 Hz, Ar-H), 9.20 (d, 4H, J = 7.1 Hz, Ar-H);13C NMR (75 MHz, MeOD-d4, 25 °C): δ; 116.4, 125.0, 126.7, 133.3, 144.6, 149.2, 150.3.
Synthesis of TpV film 1
TpV Film was synthesized in a 25 mL glass vial, 15.7 mg (0.075 mmol) of 1,3,5-triformylphloroglucinol (Tp) (15.7 mg) dissolved in 10 mL of dichloromethane was poured into the glass vial. A spacer layer of 5 mL of water was added on top of the aldehyde solution. To the top of organic layer, aqueous solution (10 mL) of 1,1'-bis(4-aminophenyl)-[4,4'-bipyridine]-1,1'-diium dichloride (V•2Cl, 45.9 mg, 0.112 mmol) and the catalyst PTSA (38.5 mg, 0.224 mmol) was added over a period of 30 min. The system was kept at room temperature for 72 h in undisturbed condition. The thin films formed at the interface were collected by removing the top aqueous layer with a dropper and washed with water, dimethylacetamide (DMAc), and acetone to purify the TpV and dried in vacuum for 30 min.
Synthesis of TpVCB[7] film 2
TpVCB[7] Film was synthesized in a 25 glass vial. 0.075 mmol of 1,3,5-triformylphloroglucinol (Tp) (15.7 mg) dissolved in 10 mL of dichloromethane was poured into the glass vial. A spacer layer of 5 mL of water was added on top of the aldehyde solution. To the top of the organic layer, aqueous solution of (10 mL) of V•2Cl (20.5 mg, 0.05 mmol) in presence of CB[7] (58.1 mg, 0.05 mmol) and PTSA (38.5 mg, 0.224 mmol) was added over a period of 30 min. The system was kept at room temperature for 72 h in undisturbed condition. The thin films formed at the interface were collected by removing the top aqueous layer with a dropper and washed with water, dimethylacetamide (DMAc), and acetone to purify the TpV CB[7]and dried in vacuum for 30 min.
Estimation of CB[7] ratio
The ratio of CB[7] per strand were determined using 13C direct excitation MAS NMR experiment. The areas under the carbonyl carbon of the TpV and the CH carbon from the CB[7] were used for the calculation (the 13C MAS spectrum with the integration is shown in Fig. S2). The area under the CO peak was normalized to 3.0 since each TpV strand contains three CO carbons and the corresponding integral value of the CH carbon of CB[7] is 10.2. Since each CB[7] molecule contains 14 CH carbons, we could readily calculate the ratio of CB[7] per strand by dividing the integral value by the number of carbons in the corresponding unit, which gives us the ratio 3/3:10.2/14 = 1:0.73~1:1.
Data availability
All data are available from the authors upon reasonable request.
References
Sauvage, J. P. Interlacing molecular threads on transition metals: catenands, catenates, and knots. Acc. Chem. Res. 23, 319–327 (1990).
Amabilino, D. B. & Stoddart, J. F. Interlocked and intertwined structures and superstructures. Chem. Rev. 95, 2725–2828 (1995).
Nepogodiev, S. A. & Stoddart, J. F. Cyclodextrin-based catenanes and rotaxanes. Chem. Rev. 98, 1959–1976 (1998).
Denis, M. & Goldup, S. M. The active template approach to interlocked molecules. Nat. Rev. Chem. 1, 0061 (2017).
Vögtle, F., Dünnwald, T. & Schmidt, T. Catenanes and rotaxanes of the amide type. Acc. Chem. Res. 29, 451–460 (1996).
Raymo, F. M. & Stoddart, J. F. Interlocked macromolecules. Chem. Rev. 99, 1643–1664 (1999).
Gil-Ramírez, G., Leigh, D. A. & Stephens, A. J. Catenanes: fifty years of molecular links. Angew. Chem. Int. Ed. 54, 6110–6150 (2015).
Evans, N. H. & Beer, P. D. Progress in the synthesis and exploitation of catenanes since the Millennium. Chem. Soc. Rev. 43, 4658–4683 (2014).
Ayme, J.-F., Beves, J. E., Campbell, C. J. & Leigh, D. A. Template synthesis of molecular knots. Chem. Soc. Rev. 42, 1700–1712 (2013).
Prakasam, T. et al. Simultaneous self-assembly of a [2]catenane, a trefoil knot, and a solomon link from a simple pair of ligands. Angew. Chem. Int. Ed. 52, 9956–9960 (2013).
Mitra, S. et al. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs). J. Am. Chem. Soc. 138, 2823–2828 (2016).
Chen, H. et al. Cationic covalent organic framework nanosheets for fast Li-ion conduction. J. Am. Chem. Soc. 140, 896–899 (2018).
Ma, H. et al. Cationic covalent organic frameworks: a simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 138, 5897–5903 (2016).
Huang, N., Wang, P., Addicoat, M. A., Heine, T. & Jiang, D. Ionic covalent organic frameworks: design of a charged interface aligned on 1D channel walls and its unusual elECTROSTATIC FUNctions. Angew. Chem. Int. Ed. 56, 4982–4986 (2017).
Yu, S.-B. et al. A polycationic covalent organic framework: a robust adsorbent for anionic dye pollutants. Polym. Chem. 7, 3392–3397 (2016).
Du, Y. et al. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 55, 1737–1741 (2016).
Zhang, W., Zhang, L., Zhao, H., Li, B. & Ma, H. A two-dimensional cationic covalent organic framework membrane for selective molecular sieving. J. Mat. Chem. A 6, 13331–13339 (2018).
Das, G. et al. Viologen-based conjugated covalent organic networks via zincke reaction. J. Am. Chem. Soc. 139, 9558–9565 (2017).
Waller, P. J., Gándara, F. & Yaghi, O. M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 48, 3053–3063 (2015).
Mal, A. et al. Supramolecular reassembly of self-exfoliated ionic covalent organic nanosheets for label-free detection of double-stranded DNA. Angew. Chem. Int. Ed. 57, 8443–8447 (2018).
Wessels, H. R., Slebodnick, C. & Gibson, H. W. Viologen-based rotaxanes from dibenzo-30-crown-10. J. Am. Chem. Soc. 140, 7358–7370 (2018).
Wang, K. et al. Highly effective binding of viologens by p-sulfonatocalixarenes for the treatment of viologen poisoning. J. Med. Chem. 52, 6402–6412 (2009).
Harada, A., Takashima, Y. & Nakahata, M. Supramolecular polymeric materials via cyclodextrin–guest interactions. Acc. Chem. Res. 47, 2128–2140 (2014).
Moon, K. & Kaifer, A. E. Modes of binding interaction between viologen guests and the cucurbit[7]uril host. Org. Lett. 6, 185–188 (2004).
Zhang, W. et al. Single-molecule conductance of viologen–cucurbit[8]uril host–guest complexes. ACS Nano 10, 5212–5220 (2016).
Tian, J. et al. Three-dimensional periodic supramolecular organic framework ion sponge in water and microcrystals. Nat. Commun. 5, 5574 (2014).
Zhang, K.-D. et al. Toward a single-layer two-dimensional honeycomb supramolecular organic framework in water. J. Am. Chem. Soc. 135, 17913–17918 (2013).
Li, H. et al. Viologen-mediated assembly of and sensing with carboxylatopillar[5]arene-modified gold nanoparticles. J. Am. Chem. Soc. 135, 1570–1576 (2013).
Buyukcakir, O., Je, S. H., Talapaneni, S. N., Kim, D. & Coskun, A. Charged covalent triazine frameworks for CO2 capture and conversion. ACS Appl. Mater. Interfaces 9, 7209–7216 (2017).
Samanta, P. et al. Chemically stable ionic viologen-organic network: an efficient scavenger of toxic oxo-anions from water. Chem. Sci. 9, 7874–7881 (2018).
Rapakousiou, A. et al. Liquid/liquid interfacial synthesis of a click nanosheet. Chem. Eur. J. 23, 8443–8449 (2017).
Dey, K. et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 139, 13083–13091 (2017).
Matsumoto, M. et al. Lewis-acid-catalyzed interfacial polymerization of covalent organic framework films. Chemistry 4, 308–317 (2018).
Lee, J. W., Samal, S., Selvapalam, N., Kim, H.-J. & Kim, K. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc. Chem. Res. 36, 621–630 (2003).
Kim, K. Mechanically interlocked molecules incorporating cucurbituril and their supramolecular assemblies. Chem. Soc. Rev. 31, 96–107 (2002).
Zhu, L., Yan, H., Wang, X.-J. & Zhao, Y. Light-controllable cucurbit[7]uril-based molecular shuttle. J. Org. Chem. 77, 10168–10175 (2012).
Song, N. & Yang, Y.-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 44, 3474–3504 (2015).
Wei, P., Yan, X. & Huang, F. Supramolecular polymers constructed by orthogonal self-assembly based on host–guest and metal–ligand interactions. Chem. Soc. Rev. 44, 815–832 (2015).
Freitag, M., Gundlach, L., Piotrowiak, P. & Galoppini, E. Fluorescence enhancement of Di-p-tolyl viologen by complexation in cucurbit[7]uril. J. Am. Chem. Soc. 134, 3358–3366 (2012).
Cacialli, F. et al. Cyclodextrin-threaded conjugated polyrotaxanes as insulated molecular wires with reduced interstrand interactions. Nat. Mater. 1, 160 (2002).
Vukotic, V. N. et al. Mechanically interlocked linkers inside metal–organic frameworks: effect of ring size on rotational dynamics. J. Am. Chem. Soc. 137, 9643–9651 (2015).
Zhu, K., Vukotic, V. N., O’Keefe, C. A., Schurko, R. W. & Loeb, S. J. Metal–organic frameworks with mechanically interlocked pillars: controlling ring dynamics in the solid-state via a reversible phase change. J. Am. Chem. Soc. 136, 7403–7409 (2014).
Zhu, K., O'Keefe, C. A., Vukotic, V. N., Schurko, R. W. & Loeb, S. J. A molecular shuttle that operates inside a metal–organic framework. Nat. Chem. 7, 514 (2015).
Park, K.-M., Whang, D., Lee, E., Heo, J. & Kim, K. Transition metal ion directed supramolecular assembly of one- and two-dimensional polyrotaxanes incorporating cucurbituril. Chem. Eur. J. 8, 498–508 (2002).
Kim, J. et al. New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 122, 540–541 (2000).
Benyettou, F. et al. Redox-responsive viologen-mediated self-assembly of CB[7]-modified patchy particles. Langmuir 32, 7144–7150 (2016).
Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19524–19527 (2012).
Benyettou, F. et al. Viologen-templated arrays of cucurbit[7]uril-modified iron-oxide nanoparticles. Chem. Eur. J. 21, 4607–4613 (2015).
Karak, S. et al. Constructing ultraporous covalent organic frameworks in seconds via an organic terracotta process. J. Am. Chem. Soc. 139, 1856–1862 (2017).
Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007).
Backes, C. et al. Spectroscopic metrics allow in situ measurement of mean size and thickness of liquid-exfoliated few-layer graphene nanosheets. Nanoscale 8, 4311–4323 (2016).
López-Díaz, D., López Holgado, M., García-Fierro, J. L. & Velázquez, M. M. Evolution of the Raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 121, 20489–20497 (2017).
Singh, A., Yip, W.-T. & Halterman, R. L. Fluorescence-on response via CB7 binding to viologen–dye pseudorotaxanes. Org. Lett. 14, 4046–4049 (2012).
Pfreundschuh, M., Martinez-Martin, D., Mulvihill, E., Wegmann, S. & Muller, D. J. Multiparametric high-resolution imaging of native proteins by force-distance curve–based AFM. Nat. Protoc. 9, 1113 (2014).
Amo, C. A. & Garcia, R. Fundamental high-speed limits in single-molecule, single-cell, and nanoscale force spectroscopies. ACS Nano 10, 7117–7124 (2016).
Dufrêne, Y. F., Martínez-Martín, D., Medalsy, I., Alsteens, D. & Müller, D. J. Multiparametric imaging of biological systems by force-distance curve–based AFM. Nat. Methods 10, 847 (2013).
Lemanis, R. & Zlotnikov, I. Finite element analysis as a method to study molluscan shell mechanics. Adv. Eng. Mater. 20, 1700939 (2018).
Biedermann, F. & Scherman, O. A. Cucurbit[8]uril mediated donor–acceptor ternary complexes: a model system for studying charge-transfer interactions. J. Phys. Chem. B 116, 2842–2849 (2012).
Metz, G., Wu, X. L. & Smith, S. O. Ramped-amplitude cross polarization in magic-angle-spinning NMR. J. Magn. Reson. Ser. A 110, 219–227 (1994).
Morcombe, C. R. & Zilm, K. W. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 162, 479–486 (2003).
Oliver, W. C. & Pharr, G. M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564–1583 (2011).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1 (1995).
Mayo, S. L. O. B. D. & Goddard, W. A. DREIDING: a generic force field for molecular simulations. J. Phys. Chem. 94, 8897 (1990).
Darden, T., York, D. & Pedersen, L. Particle Mesh Ewald—an N.Log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Nosé, S. A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Acknowledgements
The research described here was sponsored by New York University Abu Dhabi (NYUAD), UAE, which we thank for its generous support of the research program at NYUAD. The research was carried out using the Core Technology Platform resources at NYUAD. Calculations were carried out on the High Performance Computing resources at New York University Abu Dhabi and AD181 faculty research grant to S.K. F.G. acknowledges the Spanish Ministry of Economy and Competitiveness for funding through the “Ramón y Cajal” and “Retos de la Sociedad (CTQ2017–87262-R)” programs.
Author information
Authors and Affiliations
Contributions
G.D., T.P. and A.T. conceived and designed the experiments. G.D. and T.P. synthesized the starting materials. G.D. performed the interfacial synthesis of the films and carried out all structural characterizations. S.K.S. and R.J. performed the AFM study and characterized the mechanical properties of the films. R.P. performed the HRTEM analysis. F.G., S.K. and N.A. performed the computational study. R.M. and M.B. performed the solid-state NMR experiments and analyzed the data. J.-C.O. was involved in the analysis of the data and the preparation of the manuscript. N.S. performed the photoluminescent experiments. G.D., A.T. and J.-C.O. wrote the manuscript, and all authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das, G., Sharma, S.K., Prakasam, T. et al. A polyrotaxanated covalent organic network based on viologen and cucurbit[7]uril. Commun Chem 2, 106 (2019). https://doi.org/10.1038/s42004-019-0207-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-019-0207-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.