Abstract
Chronic hepatitis B virus (HBV) infection is a serious problem owing to its worldwide distribution and potential adverse sequelae that include cirrhosis and/or hepatocellular carcinoma. Current antiviral therapies have much improved outcomes, but few patients achieve the ultimate goal of hepatitis B surface antigen (HBsAg) loss (functional cure). As hepatitis B e antigen (HBeAg)-negative chronic HBV infection is the final phase prior to HBsAg loss, the management of patients in this phase together with quantification of HBsAg has attracted increasing clinical and research interest. This Review integrates the findings from research in HBsAg kinetics and discusses how they might inform our understanding and management of HBeAg-negative chronic HBV infection. Studies have shown that HBsAg levels are highly predictive of the presence of inactive HBV infection and that serial changes in HBsAg levels might predict HBsAg loss within 1–3 years. Data also suggest that quantitative HBsAg monitoring is important during hepatitis flare and antiviral therapy, especially in the timing of the decision to stop therapy and to start off-therapy retreatment. These findings have shed new light on the natural course of HBV infection and might lead to optimization of the management of HBeAg-negative chronic HBV infection and contribute to the paradigm shift from indefinite to finite therapy for patients with HBV infection.
Key points
-
Chronic hepatitis B virus (HBV) infection is a serious clinical problem involving ~292 million people worldwide, and has the potential risk of adverse sequelae including cirrhosis and hepatocellular carcinoma.
-
A hepatitis B surface antigen (HBsAg) level <1,000 IU/ml is indicative of inactive infection and a level <200 IU/ml with decline of >0.5 log10 IU/ml per year predicts HBsAg loss within 1–3 years.
-
A hepatitis B e antigen (HBeAg)-negative patient with active infection requires nucleoside or nucleotide analogue (NUC) or interferon-based therapy; interferon-based therapy increases HBsAg decline and rate of loss.
-
Rapid HBsAg decline of >0.5 log10 IU/ml in 6 months can lead to HBsAg levels of <100 IU/ml or HBsAg loss during NUC therapy.
-
Stopping NUC therapy in sustained responders and those who achieve HBsAg <100 IU/ml by the end of therapy might increase the 5-year HBsAg loss by up to >30%.
-
HBsAg kinetics during clinical relapse can help the retreatment decision; retreatment is required in those with increasing HBsAg levels, whereas retreatment might be unnecessary in those with decreasing HBsAg levels.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 3, 383–403 (2018).
Liaw, Y. F. & Chu, C. M. Hepatitis B virus infection. Lancet 373, 582–592 (2009). A comprehensive review on the natural history and therapy of chronic HBV infection.
Chen, Y. C. & Liaw, Y. F. Pharmacotherapeutic options for hepatitis B. Expert. Opin. Pharmacother. 17, 355–367 (2016).
Chang, M. L. & Liaw, Y. F. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course and management. J. Hepatol. 61, 1407–1417 (2014). A review of hepatitis B flare, a frequent immune-mediated event that might lead to sustained remission or disease progression.
Liaw, Y. F. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology 54, E1–E9 (2011).
Brunetto, M. R. A new role for an old marker, HBsAg. J. Hepatol. 52, 475–477 (2010).
Chan, H. L. et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology 52, 1232–1241 (2010).
Nguyen, T. et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J. Hepatol. 52, 508–513 (2010).
Jaroszewicz, J. et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J. Hepatol. 52, 514–522 (2010).
Sarin, S. K. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10, 1–98 (2016).
European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 67, 370–398 (2017).
Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67, 1560–1599 (2018).
Brunetto, M. R. et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology 139, 483–490 (2010). A landmark study on the HBsAg level of <1,000 IU/ml for the identification of carriers of inactive HBV.
Tai, D. I. et al. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology 49, 1859–1867 (2009).
Chu, C. M., Chen, Y. C., Tai, D. I. & Liaw, Y. F. Level of hepatitis B virus DNA in inactive carriers with persistently normal levels of alanine aminotransferase. Clin. Gastroenterol. Hepatol. 8, 535–540 (2010).
Chen, Y. C., Huang, S. F., Chu, C. M. & Liaw, Y. F. Serial HBV DNA levels in patients with persistently normal transaminase over 10 years following spontaneous HBeAg seroconversion. J. Viral Hepat. 19, 138–146 (2012).
Oliveri, F. et al. Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis B virus infection: benign course towards HBsAg clearance. Liver Int. 37, 1622–1631 (2017).
Bonacci, M. et al. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. Aliment. Pharmacol. Ther. 47, 1397–1408 (2018).
Liu, J. et al. Serum levels of hepatitis B surface antigen and DNA can predict inactive carriers with low risk of disease progression. Hepatology 64, 381–389 (2016).
Tseng, T. C. et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 57, 441–450 (2013).
Brouwer, W. P. et al. Repeated measurements of hepatitis B surface antigen identify carriers of inactive HBV during long-term follow-up. Clin. Gastroenterol. Hepatol. 14, 1481–1489 (2016).
Pfefferkorn, M. et al. Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers. Gut 67, 2045–2053 (2018).
Martinot-Peignoux, M. et al. Prediction of disease reactivation in asymptomatic hepatitis B e antigen-negative chronic hepatitis B patients using baseline serum measurements of HBsAg and HBV-DNA. J. Clin. Virol. 58, 401–407 (2013).
Zhu, L. et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance and seroconversion in hepatitis B e antigen-negative chronic infection patients: a population-based prospective cohort. J. Viral Hepat. 25, 1588–1598 (2018).
Chan, H. L., Wong, G. L., Tse, C. H., Chan, H. Y. & Wong, V. W. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J. Infect. Dis. 204, 408–414 (2011).
Tseng, T. C. et al. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology 141, 517–525 (2011).
Chen, Y. C., Jeng, W. J., Chu, C. M. & Liaw, Y. F. Decreasing levels of HBsAg predict HBsAg seroclearance in patients with inactive chronic hepatitis B virus infection. Clin. Gastroenterol. Hepatol. 10, 297–302 (2012). A long-term HBsAg kinetic study in carriers of inactive HBV that led to the proposal of criteria for the prediction of HBsAg loss in 1–3 years.
Liu, J. et al. A predictive scoring system for the seroclearance of HBsAg in HBeAg-seronegative chronic hepatitis B patients with genotype B or C infection. J. Hepatol. 58, 853–860 (2013).
Seto, W. K. et al. A large case-control study on the predictability of hepatitis B surface antigen levels three years before hepatitis B surface antigen seroclearance. Hepatology 56, 812–819 (2012).
Bonino, F. & Brunetto, M. R. Chronic hepatitis B e antigen (HBeAg) negative, anti-HBe positive hepatitis B: an overview. J. Hepatol. 39, (Suppl. 1), S160–S163 (2003).
Liaw, Y. F., Tai, D. I., Chu, C. M., Pao, C. C. & Chen, T. J. Acute exacerbation in chronic type B hepatitis: comparison between HBeAg and antibody-positive patients. Hepatology 7, 20–23 (1987).
Liaw, Y. F. et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol. Int. 6, 531–561 (2012). Guidelines that propose a stopping rule for nucleoside or nucleotide therapy in HBV e antigen (HBeAg)-negative patients with chronic HBV infection.
Liaw, Y. F., Pao, C. C. & Chu, C. M. Changes of serum HBV-DNA in relation to serum transaminase level during acute exacerbation in patients with chronic type B hepatitis. Liver 8, 231–235 (1988).
Levrero, M. et al. Control of cccDNA function in hepatitis B virus infection. J. Hepatol. 51, 581–592 (2009).
Papatheodoridis, G. et al. Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis B patients under 4-year entecavir therapy. J. Hepatol. 60, 62–68 (2014).
Papatheodoridis, G. et al. Serum HBsAg kinetics and usefulness of interferon-inducible protein 10 serum in HBeAg-negative chronic hepatitis B patients treated with tenofovir disoproxil fumarate. J. Viral Hepat. 22, 1079–1087 (2015).
Chevaliez, S., Hézode, C., Bahrami, S., Grare, M. & Pawlotsky, J. M. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J. Hepatol. 58, 676–683 (2013). A study showing why several decades is required for nucleoside or nucleotide therapy to achieve the therapeutic goal of HBV surface antigen loss.
Marcellin, P. et al. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 150, 134–144 (2016).
Marcellin, P. et al. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol. Int. 7, 88–97 (2013).
Brunetto, M. R. et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 49, 1141–1150 (2009).
Lampertico, P. et al. Randomised study comparing 48 and 96 weeks peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 62, 290–298 (2013).
Moucari, R. et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology 49, 1151–1157 (2009).
Peng, C. Y. et al. Early serum HBsAg level as a strong predictor of sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Aliment. Pharmacol. Ther. 35, 458–468 (2012).
Lee, I. C. et al. Incidence and predictors of HBsAg loss after peginterferon therapy in HBeAg-negative chronic hepatitis B: a multicenter, long-term follow-up study. J. Infect. Dis. 218, 1075–1084 (2018).
Chuaypen, N. et al. Predictive role of serum HBsAg and HBcrAg kinetics in patients with HBeAg-negative chronic hepatitis B receiving pegylated interferon-based therapy. Clin. Microbiol. Infect. 24, 306.e7–306.e13 (2018).
Rijckborst, V. et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J. Hepatol. 56, 1006–1011 (2012).
Boglione, L., Cusato, J., Cariti, G., Di Perri, G. & D’Avolio, A. Role of HBsAg decline in patients with chronic hepatitis B HBeAg-negative and E genotype treated with pegylated-interferon. Antivir. Res. 136, 32–36 (2016).
Moucari, R. et al. Influence of genotype on hepatitis B surface antigen kinetics in hepatitis B e antigen-negative patients treated with pegylated interferon-alpha2a. Antivir. Ther. 14, 1183–1188 (2009).
Brunetto, M. R. et al. Response to peginterferon alfa-2a (40KD) in HBeAg-negative CHB: on-treatment kinetics of HBsAg serum levels vary by HBV genotype. J. Hepatol. 59, 1153–1159 (2013).
Buti, M. et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. Gastroenterol. Hepatol. 1, 196–206 (2016).
Wong, G. L. et al. Serum hepatitis B surface antigen kinetics in severe reactivation of hepatitis B e antigen negative chronic hepatitis B patients receiving nucleoside/nucleotide analogues. Antivir. Ther. 18, 979–986 (2013).
Zoulim, F. et al. Quantification of HBsAg in nucleos(t)ide-naïve patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J. Hepatol. 62, 56–63 (2015).
Jeng, W. J., Chen, Y. C., Chang, M. L. & Liaw, Y. F. α-Fetoprotein level-dependent early HBsAg decline during entecavir therapy in chronic hepatitis B with hepatitis flare. J. Antimicrob. Chemother. 71, 1601–1608 (2016).
Liaw, Y. F., Tai, D. Y., Chen, T. J., Chu, C. M. & Huang, M. J. Alpha-fetoprotein changes in the course of chronic hepatitis: relation to bridging hepatic necrosis and hepatocellular carcinoma. Liver 6, 133–137 (1986).
Xia, Y. et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 150, 194–205 (2016).
Boni, C. et al. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J. Hepatol. 39, 595–605 (2003).
Jeng, W. J., Chen, Y. C. & Liaw, Y. F. Great and rapid HBsAg decline in patients with on-treatment hepatitis flare in early phase of potent antiviral therapy. J. Viral Hepat. 25, 421–428 (2018).
Peng, C. Y. et al. Early hepatitis B surface antigen decline predicts treatment response to entecavir in patients with chronic hepatitis B. Sci. Rep. 7, 42879 (2017).
Kim, G. A. et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut 63, 1325–1332 (2014).
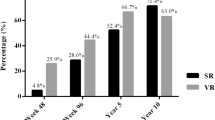
Jeng, W. J., Chen, Y. C., Chien, R. N., Sheen, I. S. & Liaw, Y. F. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology 68, 425–434 (2018). The largest study in HBeAg-negative patients showing much higher HBV surface antigen loss rate after cessation of nucleoside or nucleotide therapy in patients with sustained remission and un-retreated clinical relapse.
Buti, M. et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig. Dis. Sci. 60, 1457–1464 (2015).
Hadziyannis, E. & Laras, A. Viral biomarkers in chronic HBeAg negative HBV infection. Genes 9, 469 (2018).
Ahn, S. H. et al. Hepatitis B surface antigen loss with tenofovir disoproxil fumarate plus peginterferon alfa-2a: week 120 analysis. Dig. Dis. Sci. 63, 3487–3497 (2018).
Marcellin, P. et al. Predictors of response to tenofovir disoproxil fumarate plus peginterferon alfa-2a combination therapy for chronic hepatitis B. Aliment. Pharmacol. Ther. 44, 957–966 (2016).
Qiu, K. et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment. Pharmacol. Ther. 47, 1340–1348 (2018).
Bourlière, M. et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet. Gastroenterol. Hepatol. 2, 177–188 (2017).
Chang, M. L., Liaw, Y. F. & Hadziyannis, S. J. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment. Pharmacol. Ther. 42, 243–257 (2015).
Papatheodoridis, G. et al. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology 63, 1481–1492 (2016).
Jeng, W. J. et al. Clinical relapse after cessation of tenofovir therapy in HBeAg-negative patients. Clin. Gastroenterol. Hepatol. 14, 1813–1820 (2016).
Kuo, M. T. et al. Hepatitis B virus relapse rates in chronic hepatitis B patients who discontinue either entecavir or tenofovir. Aliment. Pharmacol. Ther. 49, 218–228 (2019).
Chen, C. H. et al. Long-term incidence and predictors of hepatitis B surface antigen loss after discontinuing nucleoside analogues in noncirrhotic chronic hepatitis B patients. Clin. Microbiol. Infect. 24, 997–1003 (2018).
Yao, C. C. et al. Incidence and predictors of HBV relapse after cessation of nucleoside analogues in HBeAg-negative patients with HBsAg ≤ 200 IU/mL. Sci. Rep. 7, 1839 (2017).
Su, T. H. et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J. Infect. Dis. 217, 1193–1201 (2018).
Höner Zu Siederdissen, C. et al. Viral and host responses after stopping long-term nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J. Infect. Dis. 214, 1492–1497 (2016).
Berg, T. et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients – FINITE study. J. Hepatol. 67, 918–924 (2017). The first and so far the only randomized controlled trial on events and increased HBsAg loss rate after stopping nucleoside or nucleotide therapy.
Höner zu Siederdissen, C. et al. Contrasting timing of virological relapse after discontinuation of tenofovir or entecavir in hepatitis B e antigen-negative patients. J. Infect. Dis. 218, 1480–1484 (2018).
Hadziyannis, S. J., Sevastianos, V., Rapti, I., Vassilopoulos, D. & Hadziyannis, E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 143, 629–636 (2012).
Chi, H. et al. Reduced risk of relapse after long-term nucleos(t)ide analogue consolidation therapy for chronic hepatitis B. Aliment. Pharmacol. Ther. 41, 867–876 (2015).
Hung, C. H. et al. Hepatitis B surface antigen loss and clinical outcomes between HBeAg-negative cirrhosis patients who discontinued or continued nucleoside analogue therapy. J. Viral Hepat. 24, 599–607 (2017).
Zimmer, C. L. et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss. J. Infect. Dis. 217, 1656–1666 (2018).
Rivino, L. et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J. Clin. Invest 128, 668–681 (2018).
Rinker, F. et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J. Hepatol. 69, 584–593 (2018).
Liaw, Y. F. Stop-and-watch strategy after cessation of nucleos(t)ide analogue therapy in HBeAg-negative patients. J. Hepatol. 68, 1102–1104 (2018).
Liaw, Y. F., Jeng, W. J. & Chang, M. L. HBsAg kinetics in re-treatment decision for off-therapy hepatitis B flare in HBeAg-negative patients. Gastroenterology 154, 2280–2281 (2018). A proof-of-concept study using combined HBsAg and alanine aminotransferase kinetics for re-treatment decision.
Jeng, W. J., Chang, M. L. & Liaw, Y. F. Off-therapy precipitous HBsAg decline predicts HBsAg loss after finite entecavir therapy in HBeAg-negative patients. J. Viral Hepat. 26, 1019–1026 (2019).
Hsu, Y. C. et al. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment. Pharmacol. Ther. 49, 107–115 (2019).
Wang, J. et al. Relationship between serum HBV-RNA levels and intrahepatic viral as well as histologic activity markers in entecavir-treated patients. J. Hepatol. 68, 16–24 (2018).
Martinez, M. G., Testoni, B. & Zoulim, F. Biological basis for functional cure of chronic hepatitis B. J. Viral Hepat. 26, 786–794 (2019).
Acknowledgements
The author has been supported by grants from Chang Gung Medical Research Fund (CMRPG1G0061-3) and the Prosperous Foundation, Taipei, Taiwan. The author thanks Su-Chiung Chu for assistance in preparing the manuscript and figures.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks N. Coppola, P. Marcellin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liaw, YF. Clinical utility of HBV surface antigen quantification in HBV e antigen-negative chronic HBV infection. Nat Rev Gastroenterol Hepatol 16, 631–641 (2019). https://doi.org/10.1038/s41575-019-0197-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0197-8
This article is cited by
-
Precision Management of Patients with HBV Infection
Current Hepatology Reports (2024)
-
When to Stop Antiviral Therapy in HBeAg-Negative Patients with Chronic Hepatitis B?
Current Hepatology Reports (2024)
-
Unusual serological profile in hepatitis B virus (HBV) infection associated with a probable clinical case of acute exacerbation of pre-existing chronic HBV infection
Molecular Biology Reports (2023)
-
Perspectives on current controversial issues in the management of chronic HBV infection
Journal of Gastroenterology (2022)
-
Evidence-Based Management of Oral Nucleos(t)ide Analogue Withdrawal in Virally Suppressed Patients with Chronic HBV Infection
Current Hepatology Reports (2022)