Abstract
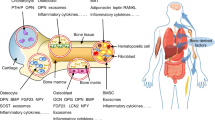
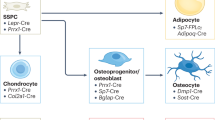
Osteoblasts are specialized mesenchymal cells that synthesize bone matrix and coordinate the mineralization of the skeleton. These cells work in harmony with osteoclasts, which resorb bone, in a continuous cycle that occurs throughout life. The unique function of osteoblasts requires substantial amounts of energy production, particularly during states of new bone formation and remodelling. Over the last 15 years, studies have shown that osteoblasts secrete endocrine factors that integrate the metabolic requirements of bone formation with global energy balance through the regulation of insulin production, feeding behaviour and adipose tissue metabolism. In this article, we summarize the current understanding of three osteoblast-derived metabolic hormones (osteocalcin, lipocalin and sclerostin) and the clinical evidence that suggests the relevance of these pathways in humans, while also discussing the necessity of specific energy substrates (glucose, fatty acids and amino acids) to fuel bone formation and promote osteoblast differentiation.
Key points
-
Osteoblasts are bone-forming cells that respond to metabolic hormones and produce at least three endocrine factors that influence whole-body metabolism.
-
Osteocalcin acts via a feedforward endocrine loop to regulate pancreatic insulin production and insulin sensitivity.
-
Osteoblast-derived lipocalin regulates feeding behaviour.
-
Sclerostin exerts control over bone tissue acquisition while also regulating WNT signalling and fatty acid synthesis in adipose tissue depots.
-
The utilization of glucose, fatty acids and amino acids by the osteoblast is associated with the stage of differentiation and the energetic demands for matrix production.
-
Key osteoblast developmental signals, including WNT–β-catenin, Notch and HIF, coordinate osteoblastic activity and intermediary metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seeman, E. Bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 19, 219–233 (2009).
Eriksen, E. F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 11, 219–227 (2010).
Martin, R. B., Burr, D. B., Sharkey, N. A. & Fyhrie, D. P. in Skeletal Tissue Mechanics (Springer, 2015).
Xian, L. et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 18, 1095–1101 (2012).
Tang, Y. et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 15, 757–765 (2009).
Pritchard, J. J. A cytological and histochemical study of bone and cartilage formation in the rat. J. Anat. 86, 259–277 (1952).
Dallas, S. L., Prideaux, M. & Bonewald, L. F. The osteocyte: an endocrine cell…. and more. Endocr. Rev. 34, 658–690 (2013).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Dobnig, H. & Turner, R. T. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 136, 3632–3638 (1995).
Dudley, H. R. & Spiro, D. The fine structure of bone cells. J. Biophys. Biochem. Cytol. 11, 627–649 (1961).
Guntur, A. R., Le, P. T., Farber, C. R. & Rosen, C. J. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology 155, 1589–1595 (2014). This study tracks the changes in oxygen consumption and glycolytic metabolism that occur during osteoblast differentiation in vitro.
Komarova, S. V., Ataullakhanov, F. I. & Globus, R. K. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am. J. Physiol. Cell Physiol. 279, C1220–C1229 (2000).
Rolfe, D. F. & Brown, G. C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 (1997).
Buttgereit, F. & Brand, M. D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 312, 163–167 (1995).
Devlin, M. J. et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Min. Res. 25, 2078–2088 (2010).
Miller, K. K. et al. Determinants of skeletal loss and recovery in anorexia nervosa. J. Clin. Endocrinol. Metab. 91, 2931–2937 (2006).
Lecka-Czernik, B. & Rosen, C. J. Energy excess, glucose utilization, and skeletal remodeling: new insights. J. Bone Min. Res. 30, 1356–1361 (2015).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000). This work is a catalyst for the exploration of metabolic functions of the skeleton.
Kajimura, D. et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 17, 901–915 (2013).
Ferron, M. et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 (2010).
Fulzele, K. et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010). This study, along with Ferron et al. ( Cell , 2010), outlines a bone–pancreas endocrine loop.
Neuman, M. W. & Neuman, W. F. Emerging concepts of the structure and metabolic functions of bone. Am. J. Med. 22, 123–131 (1957).
Neuman, W. F., Neuman, M. W. & Brommage, R. Aerobic glycolysis in bone: lactate production and gradients in calvaria. Am. J. Physiol. 234, C41–C50 (1978).
Celeste, A. J. et al. Isolation of the human gene for bone gla protein utilizing mouse and rat cDNA clones. EMBO J. 5, 1885–1890 (1986).
Puchacz, E. et al. Chromosomal localization of the human osteocalcin gene. Endocrinology 124, 2648–2650 (1989).
Hauschka, P. V., Lian, J. B., Cole, D. E. & Gundberg, C. M. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 69, 990–1047 (1989).
Price, P. A., Otsuka, A. A., Poser, J. W., Kristaponis, J. & Raman, N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl Acad. Sci. USA 73, 1447–1451 (1976).
Ducy, P. et al. Increased bone formation in osteocalcin-deficient mice. Nature 382, 448–452 (1996).
Boskey, A. L. et al. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23, 187–196 (1998).
Murshed, M., Schinke, T., McKee, M. D. & Karsenty, G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J. Cell Biol. 165, 625–630 (2004).
Poundarik, A. A. et al. Dilatational band formation in bone. Proc. Natl Acad. Sci. USA 109, 19178–19183 (2012).
Nikel, O., Laurencin, D., McCallum, S. A., Gundberg, C. M. & Vashishth, D. NMR investigation of the role of osteocalcin and osteopontin at the organic–inorganic interface in bone. Langmuir 29, 13873–13882 (2013).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007). This work provides the first evidence that osteocalcin acts as a hormone.
Ferron, M., McKee, M. D., Levine, R. L., Ducy, P. & Karsenty, G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50, 568–575 (2011).
Ferron, M., Hinoi, E., Karsenty, G. & Ducy, P. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl Acad. Sci. USA 105, 5266–5270 (2008).
Sabek, O. M. et al. Osteocalcin effect on human β-cells mass and function. Endocrinology 156, 3137–3146 (2015).
Clemens, T. L. & Karsenty, G. The osteoblast: an insulin target cell controlling glucose homeostasis. J. Bone Min. Res. 26, 677–680 (2011).
Rached, M. T. et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J. Clin. Invest. 120, 357–368 (2010).
Pi, M., Wu, Y. & Quarles, L. D. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J. Bone Min. Res. 26, 1680–1683 (2011).
Wei, J., Hanna, T., Suda, N., Karsenty, G. & Ducy, P. Osteocalcin promotes β-cell proliferation during development and adulthood through Gprc6a. Diabetes 63, 1021–1031 (2014).
Hawley, J. A., Hargreaves, M., Joyner, M. J. & Zierath, J. R. Integrative biology of exercise. Cell 159, 738–749 (2014).
Mera, P. et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 23, 1078–1092 (2016).
Mera, P., Laue, K., Wei, J., Berger, J. M. & Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 5, 1042–1047 (2016). This study, along with Mera et al. ( Cell Metab . , 2016), highlights the effects of osteocalcin on skeletal muscle.
Gundberg, C. M., Nieman, S. D., Abrams, S. & Rosen, H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J. Clin. Endocrinol. Metab. 83, 3258–3266 (1998).
Ferron, M., Wei, J., Yoshizawa, T., Ducy, P. & Karsenty, G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem. Biophys. Res. Commun. 397, 691–696 (2010).
Kunutsor, S. K., Apekey, T. A. & Laukkanen, J. A. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur. J. Epidemiol. 30, 599–614 (2015).
Liu, J. M. et al. An independent positive relationship between the serum total osteocalcin level and fat-free mass in healthy premenopausal women. J. Clin. Endocrinol. Metab. 98, 2146–2152 (2013).
Liu, C. et al. Association between serum total osteocalcin level and type 2 diabetes mellitus: a systematic review and meta-analysis. Horm. Metab. Res. 47, 813–819 (2015).
Confavreux, C. B. et al. Lower serum osteocalcin is associated with more severe metabolic syndrome in elderly men from the MINOS cohort. Eur. J. Endocrinol. 171, 275–283 (2014).
Saleem, U., Mosley, T. H. Jr. & Kullo, I. J. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 30, 1474–1478 (2010).
Yeap, B. B. et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur. J. Endocrinol. 163, 265–272 (2010).
Yeap, B. B. et al. Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J. Clin. Endocrinol. Metab. 100, 63–71 (2015).
Gower, B. A. et al. Associations of total and undercarboxylated osteocalcin with peripheral and hepatic insulin sensitivity and β-cell function in overweight adults. J. Clin. Endocrinol. Metab. 98, E1173–E1180 (2013).
Iki, M. et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos. Int. 23, 761–770 (2012).
Bonneau, J. et al. Association between osteocalcin γ-carboxylation and insulin resistance in overweight and obese postmenopausal women. J. Diabetes Complications 31, 1027–1034 (2017).
Basu, R., Peterson, J., Rizza, R. & Khosla, S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. J. Clin. Endocrinol. Metab. 96, 1450–1455 (2011).
Schwartz, A. V. et al. Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J. Bone Min. Res. 28, 1348–1354 (2013).
Confavreux, C. B. et al. Osteoid osteoma is an osteocalcinoma affecting glucose metabolism. Osteoporos. Int. 23, 1645–1650 (2012).
Lin, X., Brennan-Speranza, T. C., Levinger, I. & Yeap, B. B. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients 10, E847 (2018).
Yoshikawa, Y. et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J. Bone Min. Res. 26, 2012–2025 (2011). This study suggests the existence of additional osteoblast-derived hormones.
Rached, M. T. et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 11, 147–160 (2010).
Yan, Q. W. et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56, 2533–2540 (2007).
Mosialou, I. et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543, 385–390 (2017). This study describes the existence of a second hormone produced by osteoblasts.
Jun, L. S., Siddall, C. P. & Rosen, E. D. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 301, E825–E835 (2011).
Ye, D. et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J. Hepatol. 65, 988–997 (2016).
Zhang, Y. et al. Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. J. Biol. Chem. 289, 22063–22077 (2014).
Guo, H. et al. Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice. Endocrinology 154, 3525–3538 (2013).
Law, I. K. et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59, 872–882 (2010).
Guo, H. et al. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59, 1376–1385 (2010).
Mera, P., Ferron, M. & Mosialou, I. Regulation of energy metabolism by bone-derived hormones. Cold Spring Harb. Perspect. Med. 8, a031666 (2018).
Wang, Y. et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 53, 34–41 (2007).
Singh, R. G., Pendharkar, S. A., Plank, L. D. & Petrov, M. S. Role of human lipocalin proteins in abdominal obesity after acute pancreatitis. Peptides 91, 1–7 (2017).
Na, G. Y. et al. The relationship between circulating neutrophil gelatinase-associated lipocalin and early alteration of metabolic parameters is associated with dietary saturated fat intake in non-diabetic Korean women. Endocr. J. 64, 303–314 (2017).
Auguet, T. et al. Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity. 19, 2295–2300 (2011).
Catalan, V. et al. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J. Mol. Med. 87, 803–813 (2009).
Auguet, T. et al. Liver lipocalin 2 expression in severely obese women with non alcoholic fatty liver disease. Exp. Clin. Endocrinol. Diabetes 121, 119–124 (2013).
Paton, C. M. et al. Lipocalin-2 increases fat oxidation in vitro and is correlated with energy expenditure in normal weight but not obese women. Obesity 21, E640–E648 (2013).
Moester, M. J., Papapoulos, S. E., Lowik, C. W. & van Bezooijen, R. L. Sclerostin: current knowledge and future perspectives. Calcif. Tissue Int. 87, 99–107 (2010).
Brunkow, M. E. et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589 (2001).
van Bezooijen, R. L. et al. SOST expression is restricted to the great arteries during embryonic and neonatal cardiovascular development. Dev. Dyn. 236, 606–612 (2007).
Balemans, W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 (2001).
Balemans, W. et al. Identification of a 52kb deletion downstream of the SOST gene in patients with van buchem disease. J. Med. Genet. 39, 91–97 (2002).
Staehling-Hampton, K. et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van buchem disease in the dutch population. Am. J. Med. Genet. 110, 144–152 (2002).
Beighton, P., Barnard, A., Hamersma, H. & van der Wouden, A. The syndromic status of sclerosteosis and van Buchem disease. Clin. Genet. 25, 175–181 (1984).
Holdsworth, G. et al. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J. Biol. Chem. 287, 26464–26477 (2012).
Bourhis, E. et al. Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure 19, 1433–1442 (2011).
Gong, Y. et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLOS ONE 5, e12682 (2010).
Day, T. F., Guo, X., Garrett-Beal, L. & Yang, Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 (2005).
Holmen, S. L. et al. Essential role of β-catenin in postnatal bone acquisition. J. Biol. Chem. 280, 21162–21168 (2005).
Li, X. et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Min. Res. 23, 860–869 (2008).
Yee, C. S. et al. Conditional deletion of sost in MSC-derived lineages identifies specific cell-type contributions to bone mass and B-cell development. J. Bone Min. Res. 33, 1748–1759 (2018).
Clarke, B. L. & Drake, M. T. Clinical utility of serum sclerostin measurements. Bonekey Rep 2, 361 (2013).
Arasu, A. et al. Serum sclerostin and risk of hip fracture in older Caucasian women. J. Clin. Endocrinol. Metab. 97, 2027–2032 (2012).
Ardawi, M. S. et al. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the center of excellence for osteoporosis research study. J. Bone Min. Res. 27, 2592–2602 (2012).
Garcia-Martin, A. et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 97, 234–241 (2012).
Gaudio, A. et al. Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 97, 3744–3750 (2012).
Gennari, L. et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J. Clin. Endocrinol. Metab. 97, 1737–1744 (2012).
van Lierop, A. H. et al. Distinct effects of pioglitazone and metformin on circulating sclerostin and biochemical markers of bone turnover in men with type 2 diabetes mellitus. Eur. J. Endocrinol. 166, 711–716 (2012).
Heilmeier, U. et al. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos. Int. 26, 1283–1293 (2015).
Yamamoto, M., Yamauchi, M. & Sugimoto, T. Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 98, 4030–4037 (2013).
Urano, T., Shiraki, M., Ouchi, Y. & Inoue, S. Association of circulating sclerostin levels with fat mass and metabolic disease–related markers in Japanese postmenopausal women. J. Clin. Endocrinol. Metab. 97, E1473–E1477 (2012).
Sheng, Z. et al. Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south Chinese postmenopausal women. Clin. Endocrinol. 76, 797–801 (2012).
Amrein, K. et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J. Clin. Endocrinol. Metab. 97, 148–154 (2012).
Confavreux, C. B. et al. Has sclerostin a true endocrine metabolic action complementary to osteocalcin in older men? Osteoporos. Int. 27, 2301–2309 (2016).
Daniele, G. et al. Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care 38, 1509–1517 (2015).
Kim, S. P. et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc. Natl Acad. Sci. USA 114, E11238–E11247 (2017). This work suggests that sclerostin acts outside the skeleton to influence adipose tissue physiology.
Fairfield, H. et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J. Cell. Physiol. 233, 1156–1167 (2018).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016).
McClung, M. R. et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 (2014).
Jacobsen, C. M. Application of anti-sclerostin therapy in non-osteoporosis disease models. Bone 96, 18–23 (2017).
Gillespie, J. R. et al. GSK-3β function in bone regulates skeletal development, whole-body metabolism, and male life span. Endocrinology 154, 3702–3718 (2013).
Frey, J. L. et al. Wnt–Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol. Cell Biol. 35, 1979–1991 (2015).
Dirckx, N. et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J. Clin. Invest. 128, 1087–1105 (2018).
Esen, E., Lee, S. Y., Wice, B. M. & Long, F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J. Bone Min. Res. 30, 1959–1968 (2015).
Felix, R., Neuman, W. F. & Fleisch, H. Aerobic glycolysis in bone: lactic acid production by rat calvaria cells in culture. Am. J. Physiol. 234, C51–C55 (1978).
Nichols, F. C. & Neuman, W. F. Lactic acid production in mouse calvaria in vitro with and without parathyroid hormone stimulation: lack of acetazolamide effects. Bone 8, 105–109 (1987).
Kenny, A. D., Draskoczy, P. R. & Goldhaber, P. Citric acid production by resorbing bone in tissue culture. Am. J. Physiol. 197, 502–504 (1959).
Taylor, T. G. The nature of bone citrate. Biochim. Biophys. Acta 39, 148–149 (1960).
Sobel, A. E., Goldenberg, H. & Schmerzler, E. Calcification. XI. Studies of the incorporation of citrate in calcification in vitro. J. Dent. Res. 33, 497–503 (1954).
Dixon, T. F. & Perkins, H. R. Citric acid and bone metabolism. Biochem. J. 52, 260–265 (1952).
Neuman, W. F. The mechanism of parathyroid function. J. Lancet 78, 190–196 (1958).
Costello, L. C., Franklin, R. B., Reynolds, M. A. & Chellaiah, M. The important role of osteoblasts and citrate production in bone formation: ‘osteoblast citration’ as a new concept for an old relationship. Open Bone J. https://doi.org/10.2174/1876525401204010027 (2012).
Wei, J. et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell 161, 1576–1591 (2015). This study indicates that GLUT1 expression is essential for osteoblast development.
Zoch, M. L., Abou, D. S., Clemens, T. L., Thorek, D. L. & Riddle, R. C. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 4, 16004 (2016).
Augustin, R. The protein family of glucose transport facilitators: it’s not only about glucose after all. IUBMB Life 62, 315–333 (2010).
Thomas, D. M., Maher, F., Rogers, S. D. & Best, J. D. Expression and regulation by insulin of GLUT 3 in UMR 106-01, a clonal rat osteosarcoma cell line. Biochem. Biophys. Res. Commun. 218, 789–793 (1996).
Thomas, D. M., Rogers, S. D., Ng, K. W. & Best, J. D. Dexamethasone modulates insulin receptor expression and subcellular distribution of the glucose transporter GLUT 1 in UMR 106-01, a clonal osteogenic sarcoma cell line. J. Mol. Endocrinol. 17, 7–17 (1996).
Zoidis, E., Ghirlanda-Keller, C. & Schmid, C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol. Cell. Biochem. 348, 33–42 (2011).
Zoidis, E., Ghirlanda-Keller, C. & Schmid, C. Triiodothyronine stimulates glucose transport in bone cells. Endocrine 41, 501–511 (2012).
Li, Z. et al. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology 157, 4094–4103 (2016).
Simpson, I. A. et al. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 295, E242–E253 (2008).
Shepherd, P. R. et al. Distribution of GLUT3 glucose transporter protein in human tissues. Biochem. Biophys. Res. Commun. 188, 149–154 (1992).
Jahn, K. et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J. Bone Min. Res. 32, 1761–1772 (2017).
Chen, C. T., Shih, Y. R., Kuo, T. K., Lee, O. K. & Wei, Y. H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26, 960–968 (2008).
Shum, L. C., White, N. S., Mills, B. N., Bentley, K. L. & Eliseev, R. A. Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Dev. 25, 114–122 (2016).
Regard, J. B., Zhong, Z., Williams, B. O. & Yang, Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 4, a007997 (2012).
Yadav, V. K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008).
Esen, E. et al. WNT–LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 17, 745–755 (2013). This work is the first to demonstrate control of osteoblast metabolism by WNT signalling.
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Karner, C. M. et al. Wnt protein signaling reduces nuclear Acetyl-CoA levels to suppress gene expression during osteoblast differentiation. J. Biol. Chem. 291, 13028–13039 (2016).
Rodan, G. A., Rodan, S. B. & Marks, S. C. Jr. Parathyroid hormone stimulation of adenylate cyclase activity and lactic acid accumulation in calvaria of osteopetrotic (ia) rats. Endocrinology 102, 1501–1505 (1978).
Bikle, D. D. et al. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J. Bone Min. Res. 17, 1570–1578 (2002).
McCarthy, T. L., Centrella, M. & Canalis, E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology 124, 1247–1253 (1989).
Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
Yorgan, T. et al. Osteoblast-specific Notch2 inactivation causes increased trabecular bone mass at specific sites of the appendicular skeleton. Bone 87, 136–146 (2016).
Zanotti, S. et al. Sustained Notch2 signaling in osteoblasts, but not in osteoclasts, is linked to osteopenia in a mouse model of Hajdu-Cheney syndrome. J. Biol. Chem. 292, 12232–12244 (2017).
Lee, S. Y. & Long, F. Notch signaling suppresses glucose metabolism in mesenchymal progenitors to restrict osteoblast differentiation. J. Clin. Invest. 128, 5573–5586 (2018).
Zanotti, S. & Canalis, E. Parathyroid hormone inhibits Notch signaling in osteoblasts and osteocytes. Bone 103, 159–167 (2017).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Wang, Y. et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 117, 1616–1626 (2007).
Regan, J. N. et al. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc. Natl Acad. Sci. USA 111, 8673–8678 (2014).
Hu, C. J., Wang, L. Y., Chodosh, L. A., Keith, B. & Simon, M. C. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol. Cell Biol. 23, 9361–9374 (2003).
Wu, C. et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 29, 817–831 (2015).
Suchacki, K. J., Cawthorn, W. P. & Rosen, C. J. Bone marrow adipose tissue: formation, function and regulation. Curr. Opin. Pharmacol. 28, 50–56 (2016).
Adamek, G., Felix, R., Guenther, H. L. & Fleisch, H. Fatty acid oxidation in bone tissue and bone cells in culture. characterization and hormonal influences. Biochem. J. 248, 129–137 (1987).
Niemeier, A. et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone 43, 230–237 (2008). This study compares lipid uptake by the skeleton with that of other tissues.
Kim, S. P. et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight 2, 92704 (2017). This study highlights the importance of fatty acid oxidation for skeletal homeostasis.
Lau, B. Y., Cohen, D. J., Ward, W. E. & Ma, D. W. Investigating the role of polyunsaturated fatty acids in bone development using animal models. Molecules 18, 14203–14227 (2013).
Hooshmand, S. et al. Dietary L-carnitine supplementation improves bone mineral density by suppressing bone turnover in aged ovariectomized rats. Phytomedicine 15, 595–601 (2008).
Rendina-Ruedy, E., Guntur, A. R. & Rosen, C. J. Intracellular lipid droplets support osteoblast function. Adipocyte 6, 250–258 (2017).
Catherwood, B. D., Addison, J., Chapman, G., Contreras, S. & Lorang, M. Growth of rat osteoblast-like cells in a lipid-enriched culture medium and regulation of function by parathyroid hormone and 1,25-dihydroxyvitamin D. J. Bone Min. Res. 3, 431–438 (1988).
Campbell, S. E. & Febbraio, M. A. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 281, E803–E808 (2001).
Hatta, H., Atomi, Y., Shinohara, S., Yamamoto, Y. & Yamada, S. The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm. Metab. Res. 20, 609–611 (1988).
Herrero, P. et al. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J. Nucl. Cardiol. 12, 574–581 (2005).
Schilling, A. F. et al. Increased bone formation in mice lacking apolipoprotein E. J. Bone Min. Res. 20, 274–282 (2005).
Kevorkova, O. et al. Low-bone-mass phenotype of deficient mice for the cluster of differentiation 36 (CD36). PlOS ONE 8, e77701 (2013).
Riddle, R. C. et al. Lrp5 and Lrp6 exert overlapping functions in osteoblasts during postnatal bone acquisition. PlOS ONE 8, e63323 (2013).
Frey, J. L., Kim, S. P., Li, Z., Wolfgang, M. J. & Riddle, R. C. -Catenin directs long-chain fatty acid catabolism in the osteoblasts of male mice. Endocrinology 159, 272–284 (2018).
Larsson, S., Jones, H. A., Goransson, O., Degerman, E. & Holm, C. Parathyroid hormone induces adipocyte lipolysis via PKA-mediated phosphorylation of hormone-sensitive lipase. Cell Signal. 28, 204–213 (2016).
Fan, Y. et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 25, 661–672 (2017).
Maridas, D. E. et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. FASEB J, 33, 2885-2898 (2018).
Conigrave, A. D., Brown, E. M. & Rizzoli, R. Dietary protein and bone health: roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu. Rev. Nutr. 28, 131–155 (2008).
Fischer, A. Amino-acid metabolism of tissue cells in vitro. Nature 161, 1008 (1948).
Finerman, G. A. & Rosenberg, L. E. Amino acid transport in bone. Evidence for separate transport systems for neutral amino and imino acids. J. Biol. Chem. 241, 1487–1493 (1966).
Adamson, L. F. & Ingbar, S. H. Further studies of amino acid transport by embryonic chick bone. J. Biol. Chem. 242, 2646–2652 (1967).
Kim, S. G. et al. Differential expression and functional characterization of system L amino acid transporters in human normal osteoblast cells and osteogenic sarcoma cells. Anticancer Res. 26, 1989–1996 (2006).
Phang, J. M. & Downing, S. J. Amino acid transport in bone: stimulation by cyclic AMP. Am. J. Physiol. 224, 191–196 (1973).
Adamson, L. F. & Ingbar, S. H. Some properties of the stimulatory effect of thyroid hormones on amino acid transport by embryonic chick bone. Endocrinology 81, 1372–1378 (1967).
Hahn, T. J., Downing, S. J. & Phang, J. M. Insulin effect on amino acid transport in bone: dependence on protein synthesis and Na+. Am. J. Physiol. 220, 1717–1723 (1971).
Hahn, T. J., Downing, S. J. & Phang, J. M. Amino acid transport in adult diaphyseal bone: contrast with amino acid transport mechanisms in fetal membranous bone. Biochim. Biophys. Acta 183, 194–203 (1969).
Yang, X. & Karsenty, G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J. Biol. Chem. 279, 47109–47114 (2004).
Yang, X. et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin–Lowry syndrome. Cell 117, 387–398 (2004).
Felig, P. Amino acid metabolism in man. Annu. Rev. Biochem. 44, 933–955 (1975).
Fan, J. et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712 (2013).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Biltz, R. M., Letteri, J. M., Pellegrino, E. D., Palekar, A. & Pinkus, L. M. Glutamine metabolism in bone. Min. Electrolyte Metab. 9, 125–131 (1983).
Brown, P. M., Hutchison, J. D. & Crockett, J. C. Absence of glutamine supplementation prevents differentiation of murine calvarial osteoblasts to a mineralizing phenotype. Calcif. Tissue Int. 89, 472–482 (2011).
Yu, Y. et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 29, 966–978 (2019). This study highlights the importance of glutamine metabolism for lineage allocation.
Karner, C. M., Esen, E., Okunade, A. L., Patterson, B. W. & Long, F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J. Clin. Invest. 125, 551–562 (2015).
Huang, T. et al. Aging reduces an ERRα-directed mitochondrial glutaminase expression suppressing glutamine anaplerosis and osteogenic differentiation of mesenchymal stem cells. Stem Cells 35, 411–424 (2017).
Stegen, S. et al. HIF-1α promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 23, 265–279 (2016).
Acknowledgements
The authors gratefully acknowledge the work by other investigators that has not been cited in this manuscript because of space limitations. Work in the authors’ laboratories is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK099134, R.C.R) and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (BX003724, R.C.R; BX001234, T.L.C.). T.L.C is also the recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks L. Bonewald and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bone modelling
-
The deposition or resorption of bone matrix on separate bone surfaces to retain overall bone shape.
- Bone remodelling
-
The consecutive resorption and then deposition of bone matrix at the same skeletal site, which is often used to replace old or damaged tissue.
- Tricarboxylic acid (TCA) cycle
-
The series of chemical reactions in the mitochondria that liberates energy from nutrient substrates.
- WNT–β-catenin signalling
-
A conserved signalling pathway requiring a WNT ligand, Frizzled receptor and low-density lipoprotein receptor-related protein 5 (LRP5)–LRP6 co-receptor that regulates the stability of the transcription factor β-catenin.
- Calvarial cells
-
A population of cells isolated from the calvarial bones of neonatal rodents, which is enriched in osteoblastic cells.
- Warburg metabolism
-
The metabolism of glucose by glycolysis rather than oxidative phosphorylation even under aerobic conditions.
- Histone acetylation
-
The modification of lysine residues in the N-terminal tail of histones with an acetyl group to increase gene expression.
- Chylomicron
-
Lipoprotein particles produced in the gut consisting of protein, phospholipids, triglycerides and cholesterol.
- Coffin–Lowry syndrome
-
A rare genetic disorder characterized by skeletal deformities, short stature and delayed intellectual development linked to mutations in the RPS6KA3 gene.
Rights and permissions
About this article
Cite this article
Dirckx, N., Moorer, M.C., Clemens, T.L. et al. The role of osteoblasts in energy homeostasis. Nat Rev Endocrinol 15, 651–665 (2019). https://doi.org/10.1038/s41574-019-0246-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0246-y
This article is cited by
-
METTL3-mediated m6A modification increases Hspa1a stability to inhibit osteoblast aging
Cell Death Discovery (2024)
-
Myeloid-derived grancalcin instigates obesity-induced insulin resistance and metabolic inflammation in male mice
Nature Communications (2024)
-
Insights and implications of sexual dimorphism in osteoporosis
Bone Research (2024)
-
Age- and sex-specific differences in the association of serum osteocalcin and cardiometabolic risk factors in type 2 diabetes
Diabetology & Metabolic Syndrome (2023)
-
Bone marrow adiposity modulation after long duration spaceflight in astronauts
Nature Communications (2023)