Abstract
The use of natural products and their derivatives has evolved as a promising approach for the treatment of various infectious diseases, particularly to combat drug-resistant microbial strains. In addition, these natural products characterized by the presence of novel structures and mechanisms of action may provide guidance toward the development of potential new chemotherapies. In the present review, antimicrobial resistance (AMR) is briefly introduced and research focused on the identification and characterization of actinomycete metabolites for antimicrobial activity is discussed. Three compounds, i.e., walkmycin B, waldiomycin, and signamycin B, with novel mechanisms of action as histidine kinase inhibitors, were isolated from the metabolites of actinomycetes. New antituberculosis antibiotics, tuberlactomicin A and caprazamycins, were discovered, and amycolamicin was identified as an antimethicillin-resistant Staphylococus aureus antibiotic. The discovery of these compounds encourages the discovery and investigation of more natural products active against antimicrobial-resistant species, thus providing scaffold for the development of effective drugs against various AMR species.
Similar content being viewed by others
Importance of natural products in combating antimicrobial resistance (AMR)
AMR has emerged as a major threat for public health and an economic burden worldwide [1]. The graveness of the situation was highlighted in the World Health Organization (WHO) declaration of “Antimicrobial Resistance: No Action Today, No Cure Tomorrow” on World Health Day, 2011 [2]. According to a review on AMR data released by the British government, the O’Neill Commission warned that in the absence of appropriate measures to combat AMR, the global death toll will increase to 10 million worldwide by 2050 [3, 4]. In light of the global threat posed by AMR, it was a major topic of discussion at the G7 Summit in 2016, and the member states agreed to collaborate to address the issue of AMR. Research and development focused on the discovery of new antimicrobial drugs is one of the most important countermeasures listed in the global action plan to combat AMR [5].
The past 20 years have witnessed a serious withdrawal of many pharmaceutical companies from the research and development of antimicrobial drugs targeting AMR strains. Globalization of resistant bacterial strains, difficulty in the discovery of new molecules with novel action mechanisms, and the associated issues of low profitability and difficulty in reaching clinical trials have resulted in the depletion in the number of new antimicrobials in the pipeline of development. Regardless of these issues, natural products still occupy an important position as potential medicines that can treat infectious diseases, cancer, and hyperlipidemia. Newman et al. [6]. reported that natural products accounted for ~33% of small-molecule new drugs launched between 1980 and 2014. In addition, ~32% of the new drugs were developed by the leads provided by natural products; thus, in total, 65% of new medicinal drugs were derived from natural products. In particular, in the field of infectious diseases, AMR is considered to be an area where natural products can be most active, as the proportion of natural products associated with the development of commercially available antimicrobial drugs is high.
Actinomycete metabolites represent a potential source of new medicines that tackle the AMR issue and has involved in searching for compounds with new scaffolds and mechanisms of action. As a result, my research team discovered two new antituberculosis antibiotics derived from actinomycete metabolites, tubelactomicin A (1) and caprazamycins, and CPZEN-45 (7) which is semisynthetic antibiotic derived from caprazamycins.
A new set of antibiotics directed toward a histidine kinase (HK), an important component of bacterial two-component regulatory system (TCS), was also discovered. These compounds were found to have a novel mechanism of action, which differed from those of the available antibiotics. Three new antibiotics, i.e., walkmycin B (3), waldiomycin (4), and signamycin B (5), were discovered. Further, amycolamicin (6), an anti-MRSA antibiotic, has been discussed. These compounds have important information that can be instrumental in combating AMR. This review outlines these attractive natural products that were recently discovered by our team (Table 1).
Search for new antituberculosis antibiotics
Tuberculosis (TB) is one of the ten leading causes of death worldwide according to the WHO estimate in 2016. The number of new incidences of TB was estimated to be 10.4 million annually and that of deaths to be ~1.8 million. In addition, the number of new patients with multidrug-resistant TB (MDR-TB) was estimated to be 490,000, of which ~6.2% were infected with extensively drug-resistant TB (XDR-TB). This increase in the incidences of MDR-TB and XDR-TB is mainly contributed by the absence of the discovery of new medicines over the past four decades until recently. Coinfection with TB and human immunodeficiency virus (HIV) further aggravates the problem. Globally, 36.7 million people are infected with HIV, with an annual death toll of 1 million. Among these, 0.4 million (40%) patients have TB/HIV coinfection, thus making TB the single major cause of death of HIV-infected patients [7].
A search program was started by my research team focused on the discovery of new compounds from the metabolites of actinomycetes that can effectively treat MDR-TB. In this program, we basically aimed to isolate a new antibiotic with a mechanism of action completely different from those of the available anti-TB drugs. The program focused on evaluating the inhibitory activity of samples only against mycobacteria using two Mycobacterium strains: M. smegmatis and M. vaccae. Furthermore, we screened these samples for having no antimicrobial activity against other bacteria such as Staphylococcus aureus and Escherichia coli. Screening of the samples derived from the metabolites of actinomycetes provided two new antibiotics, i.e., tuberlactomicin A (1) and caprazamycins.
Tubelactomicin A (1, Fig. 1) is an antibiotic comprising a 16-membered macrocyclic lactone and decalin ring. It is derived from the Nocardia vinacea strain MK703-102F1 [8, 9]. N. vinacea is a new species belonging to the genus Nocardia [10]. Tubelactomicin A has been shown to have a strong antibacterial activity only against mycobacteria, M. tuberculosis, and rapidly growing nontuberculosis mycobacteria [8]. Tubelactomicin A showed minimum inhibitory concentrations (MICs) of 0.1 µg ml−1 against M. vaccae, 0.2 µg ml−1 against M. smegmatis, 7.8 µg ml−1 against M. tuberculosis H37Rv and Kurono strains, and <3.9 µg ml−1 against M. bovis Ravenel strain. A cross-resistance test using M. smegmatis indicated no cross-resistance of tubelactomicin A with kanamycin, viomycin, paromomycin, streptomycin (SM), streptothricin, rifampicin (RFP), and isoniazid (INH).
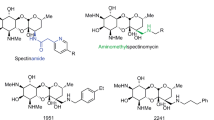
The structure of new antibiotics and their production microorganisms introduced in this review: (1) tubelactomicin A, (2) caprazamycin B, (3) walkmycin B, (4) waldiomycin, (5) signermycin B, and (6) amycolamicin. The absolute structure 3 was determined by X-ray crystallography. The X-ray crystallographic data for 3 were deposited in the Cambridge Crystallographic Data Centre under deposition number CCDC 188433
Caprazamycins belong to a group of liponucleoside antibiotics isolated from Streptomyces sp. MK730-62F2. They show strong antibacterial activity against mycobacteria and gram-positive bacteria [11, 12]. The structure of caprazamycins is characterized by a core structure of uracil, 5-aminoribose, and a cyclic peptide. In addition, side chains composed of trimethyl-rhamnoside methyl-glutarate and fatty acid chains are present. Caprazamycins have been isolated as seven components (A-G) based on the differences in side chains. While determining the stereochemical structure of caprazamycins, two core structures were isolated by acidic or alkaline treatment and were named as caprazol and caprazene (Fig. 2) [12]. Their main component, caprazamycin B (2, Fig. 1), showed an excellent in vitro antimycobacterial activity against drug-susceptible and MDR-TB strains. The MICs of caprazamycin B were 3.13 µg ml−1 against M. tuberculosis H37Rv and Kurono and 3.13 µg ml−1 against M. bovis Ravenel [12]. Furthermore, in anti-Mycobacterium avium/intracellulare activity experiments, caprazamycin B exhibited MIC values and MIC distribution curves equivalent to those of clarithromycin [11, 12]. A short-term treatment of M. tuberculosis H37Rv-infected mice with caprazamycin B at doses of 5 and 1.5 mg/kg/day by intranasal administration resulted in a dose-dependent reduction in the number of tubercle bacilli in the lungs, indicating the in vivo efficacy of caprazamycin B [13]. However, no significant signs of toxicity were observed in mice receiving a single dose (>200 mg/kg, i.v.) and repeated doses (100 mg/kg/14 days) of caprazamycin B. In addition, it was found to be well tolerated in genotoxicity and cytotoxicity tests (5000 µg ml−1) [11]. Caprazamycin B acts by inhibiting the phospho-N-acetylmuramyl pentapeptide transferase MraY involved in the cell wall biosynthesis pathway of M. tuberculosis, making it different from the available anti-TB drugs (Fig. 3) [14]. Some microbial metabolites such as liposidomycins [15, 16], capuramycin [17], RS-118641 [18], and sansanmycin [19] have been known to show strong inhibition against MraY and, thus, found to be effective against M. tuberculosis. However, none of the MraY inhibitors have been developed as pharmaceuticals yet.
The biosynthetic pathways of peptidoglycan and mycolylarabinogalactan and its inhibitors. This figure was partially modified and reprinted from a study published in J Antibiot [38]
Despite their effectiveness, the issues of high cost of separation and purification of multiple components and low water solubility associated with caparazamycins limit their pharmaceutical translation.
CPZEN-45 (7, Fig. 2) is a semisynthetic derivative of caprazamycins that overcomes the shortcomings associated with caprazamycins. It is the most promising caprazamycin derivative and shows superior antituberculosis activity. In particular, it showed antibacterial activity against slow-growing mycobacteria including M. tuberculosis. The MICs of CPZEN-45 have been reported to be 1.56 µg ml−1 against M. tuberculosis H37Rv and Kurono and 3.13 µg ml−1 against M. bovis Ravenel [20, 21]. CPZEN-45 is a compound that overcomes the aforementioned disadvantages of caprazamycins. That is, structural diversity can be converged to a common core skeleton, caprazene, by acidic treatment, and can be isolated without further separation and purification. In addition, caparzamycins generally show low water solubility owing to their amphoteric nature; however, CPZEN-45 is because the free carboxylic acid on the diazepinon ring is converted to amide and becomes a basic molecule (due to the 5-aminoribose) as a result the solubility was greatly improved.
The evaluation of in vivo efficacy in a TB mouse infection model showed CPZEN-45 to be highly effective not only toward the H37Rv strain but also toward XDR-TB as studied using a test with ten drugs. Short-term treatment of M. tuberculosis H37Rv-infected mice with CPZEN-45 demonstrated a dose-dependent reduction in the number of tubercle bacilli in the lungs. Subcutaneous administration of CPZEN-45 at a dose of 25 mg/kg/day showed therapeutic efficacy comparable to that of SM at a dose of 100 mg/kg/day in the thighs infected with the H37Rv strain (Fig. 4a). CPZEN-45 was also found to be effective in an XDR-TB-infected mice model that was resistant to INH, RFP, levofloxacin, SM, ethambutol, p-aminosalicylate, pyrazinamide, kanamycin, enviomycin, and ethionamide. Treatment of mice with CPZEN-45 at doses of 6.3, 25, 100, and 200 mg/kg/day resulted in a dose-dependent reduction in the number of viable organisms not only in the lungs (Fig. 4b) but also in the spleen and liver. CPZEN-45 also showed an excellent synergistic/additive effect when used in combination with INH, RMP, and INH + RMP. The INH + CPZEN-45 regimen was significantly more effective than the RMP + INH regime. However, the RMP + INH + CPZEN-45 regime was the most effective among all regimens, and it resulted in infection levels remarkably lower than those observed in untreated mice (Fig. 5) [21]
Therapeutic efficacy of CPZEN-45 in monotherapy and in combination with TB Drugs against M. tuberculosis H37Rv. The used amount of drug for combination-therapy as follows; rifampicin (RFP, 5 mg/kg, PO), isoniazid (INH, 1.5 mg/kg, PO), CPZEN-45-high (25 mg/kg, SC), and CPZEN-45-low (6.3 mg/kg, SC). Combination is RMP, INH, CPZEN-45-high, RFP + INH, CPZEN-45-high + RMP, CPZEN-45-high + INH, CPZEN-45-high + RMP + INH, CPZEN-45-low + RMP + INH, and CPZEN-45-low
.
Because CPZEN-45 did not show any cross-resistance with caprazamycins, it was hypothesized to have a different mechanism of action [14]. Evaluation of its activity revealed CPZEN-45 to be a mycolyl-arabinogalactan biosynthesis inhibitor (Fig. 3). The anti-TB activity of CPZEN-45 is contributed by the specific inhibition of the phospho-N-acetyl glucosaminyl transferase WecA, which is involved in galactan biosynthesis. The IC50 value against WecA has been reported to be 6.4 nM [14]. By advancing structural activity correlation studies of caprazamycins, peptidoglycan synthesis inhibitors, it led to the discovery of CPZEN-45, an inhibitor of the mycolyl-arabinogalactan biosynthesis process, with novel action point for M. tuberculosis. This interesting discovery provided a novel action point for combating M. tuberculosis. CPZEN-45 specifically acts by inhibiting WecA, a mechanism of action different from those of the existing antimicrobials and anti-TB drugs; thus, it represents a new alternative to multidrug therapy, which is the standard treatment for TB.
CPZEN-45 is under development as an MDR/XDR-TB drug via an inhalation route of administration because the preliminary in vivo kinetic studies have shown its effects to be long-lasting in the lungs. CPZEN-45 is undergoing preclinical evaluation for development as an anti-MDR/XDR-TB antibiotic as part of a collaborative development between the Institute of Microbial Chemistry (BIKAKEN) and Lilly TB Drug Discovery Initiative.
Search for HK inhibitors from actinomycetes
TCSs allow bacteria to rapidly adapt to various physical, chemical, and biological stress from outside cells. Bacterial TCSs in general comprise a membrane-bound sensor HK and cytosolic response regulator (RR) [22]. As shown in Fig. 6, upon receiving an environmental stimulus, the sensor HK first autophosphorylates a conserved histidine residue present in the dimerization domain; this is followed by the transfer of a phosphoryl group to a conserved aspartic acid residue located in the regulatory domain of the cognate RR. This phosphorylated RR further binds to the upstream promoter regions of target genes and regulates their expression. Some of these TCSs regulate the gene clusters essential for cell viability, whereas other TCSs control the genes involved in virulence, biofilm formation, and quorum sensing in pathogenic bacteria [23]. Thus, TCS inhibitors are expected to exert a wide array of effects on cell viability [23, 24]. Among the various TCSs, the WalK/WalR TCS is particularly essential for survival and is specifically associated with low GC-content gram-positive bacteria such as S. aureus [25] and Enterococcus faecalis [26]. HKs are attracting immense attention as a novel antibacterial drug target [23, 27, 28]. WalK is a membrane-linked HK comprising two domains located in the cytoplasmic C-terminal region, catalytic (kinase or ATP binding) domain and dimerization domain, characterized by the presence of a conserved phosphorylated histidine residue (H386 in Bacillus subtilis) [29, 30]. Sequence similarities indicate that WalK phosphorylation occurs at the conserved residue His387 (all amino acid sequence coordinates are those of the prototypical B. subtilis protein). Although the N-terminal domain sequence of the WalK HK shows considerable variation among species, the cytoplasmic C-terminal HK region is conserved and can be used for screening WalK inhibitors.
Paradigm of the two-component signal transduction pathways. The sensor histidine kinase (HK) autophosphorylates on a conserved histidine residue with subsequent transfer of the phosphoryl group to a conserved aspartate residue of a cognate response regulator (RR). Phosphorylated RRs are often involved in binding DNAs for the regulation of gene expression. The WalR mutant (H215P) of B. subtilis was supersensitive to inhibitors of HK
The specific hypersensitivities demonstrated by temperature-sensitive mutants facilitate the application of these mutants in whole-cell screening, providing a rapid method for the development of target-specific screens. This will be highly instrumental in the identification of novel compounds. Previously, Utsumi et al. developed a differential growth assay targeting the WalK/WalR TCS wherein a temperature-sensitive WalR mutant derived from B. subtilis was supersensitive to HK inhibitors compared with the wild-type 168 strain [31]. To isolate active inhibitors targeting WalK, a wide range of soil microbacterial culture extracts were further screened using this method. The screening of 10,000 actinomycete fermentation broths aided in the discovery of three potent WalK inhibitors.
Walkmycin B (3, Fig. 1) [32] is an antibiotic comprising a 3,3″-dianthracene and is isolated from Streptomyces sp. MK632-100F11. It is a potent inhibitor of WalK HK, which is involved in the regulation of cell division and cell wall biosynthesis. It showed excellent antibacterial activity against gram-positive bacteria including MRSA. The MICs of 3 against S. aureus 209P and S. aureus MRSA No. 5 were found to be 0.25 and 0.25 µg ml−1, respectively [32].
Furthermore, walkmycin B showed a strong HK inhibitory activity against WalK derived from B. subtilis, VicK from Streptococcus mutans, and PehS from Pectobacterium carotovorum. The IC50 values of 3 against bacterial HK were 0.69 µM for WalK, 3.4 µM for VicK, and 6.24 µM for PehS [32].
Waldiomycin (4, Fig. 1) is an angucycline antibiotic derived from Streptomyces sp. MK844-mF10. It is characterized by a planar structure similar to that of dioxamycin, except the presence of a joint at the C-D ring moiety [33]. Waldiomycin inhibits HK and shows moderate antibacterial activity against gram-positive bacteria including MRSA. The MICs of waldiomycin against S. aureus 209P and S. aureus MRSA No. 5 were found to be 8 and 16 µg ml−1, respectively [33]. Furthermore, waldiomycin shows moderate HK inhibitory activity against WalK, VicK, and PehS. The IC50 values of waldiomycin against bacterial WalK, VicK, and PehS have been reported to be 10.2, 56.2, and 50.0 µM, respectively [33]. The mechanism of action of waldiomycin against HK was analyzed using NMR spectroscopy, and it revealed that an angucyclinone moiety of waldiomycin binds to the Hbox region containing a histidine residue, which is commonly found in various bacterial HKs; this is followed by phosphorylation. Angucyclinone antibiotics, such as aquayamycin, rabelomycin, and sakyomicin A, also show antimicrobial activity against S. aureus in the same range of MIC; however, these compounds lacked inhibitory activity against HK and showed no positive response in the differential growth assay [33]. Further, a methyl ester of waldiomycin carboxylic acid at C-12″ showed no activity against HK and gram-positive bacteria. These observations indicate that the spatial arrangement of dioxolan carboxylic acid and angucycline moieties in waldiomycin might be essential for its HK inhibitory activity [34].
Signermycin B (5, Fig. 1) is an antibiotic composed of a tetramate and decalin ring and is derived from Streptomyces sp. MK851-mF8. Signermycin B acts by inhibiting HK WalK and has demonstrated a moderate antibacterial activity against gram-positive bacteria including MRSA [35]. In comparison, signermycin B failed to show any antibacterial activity against gram-negative bacteria such as P. carotovorum even at a high concentration of 128 µg ml−1. Furthermore, signermycin B shows strong HK inhibitory activity with IC50 values of 43 µM against WalK, 6.24 µM against VicK, and 15.7 µM against PehS. In particular, signermycin B showed a stronger inhibition against the HK of PehS derived from P. carotovorum, indicating the possibility of controlling virulence through PehS.
Search for new anti-Staphylococcus aureus and Enterococcus antibiotics
MRSA is one of the most problematic pathogen among the various hospital-acquired infections. At present, MRSA has developed resistance against a majority of the commercially available antibiotics, including vancomycin, which has been in use for long as a last-resort antibiotic. Only few drugs are clinically available for the treatment of vancomycin-intermediate/resistant MRSA (VISA) infections such as linezolid, quinupristin/dalfopristin, and daptomycin. Thus, the development of novel drugs effective against VISA is an active area of research. As already mentioned, MRSA is resistant to most existing drugs; thus, it is believed that the use of highly resistant MRSA strains including VISA with known resistance mechanisms can be helpful in the discovery of new compounds possessing novel mechanisms of action.
We used this method to isolate microbial metabolites with strong activity against MRSA with novel mechanisms of action.
Amycolamicin (6, Fig. 1) is an antibiotic containing a tetramate similar to that found in kibdelomycin [36] and is derived from Amycolatopsis sp. MK575-fF5 [37]. Amycolamicin is a potent and broad-spectrum antibiotic that exerts its effect by inhibiting DNA gyrase and bacterial topoisomerase IV [37]. Amycolamicin demonstrated strong antibacterial activity against gram-positive bacteria, such as MRSA, VRE, and S. pneumoniae, and gram-negative bacteria, such as Haemophilus influenzae [37].
No acute toxicity was reported in mice receiving subcutaneous injection of amycolamicin at a dose of 250 mg/kg. In addition, no genotoxicity was observed in the Ames test. The results of macromolecular synthesis studies and an enzyme assay showed that amycolamicin inhibits DNA biosynthesis. Amycolamicin inhibited DNA gyrase and topoisomerase IV, with IC50 values of 24.4 and 6250 ng ml−1, respectively [37]. No cross-resistance to amycolamicin was observed for the bacterial strains genetically induced to exhibit resistance by exposure to novobiocin and coumermycin, which are known to target gyrase subunit B. This strongly suggests that these drugs have different modes of binding to gyrase subunit B [37].
Closing remarks
A new set of antibiotics characterized by novel mechanisms of action and scaffolds were discovered and successfully isolated from actinomycete metabolites. These drugs were completely different from the commercially available antibiotics. The discovery of these novel compounds reinforces the requirement for exploring new natural drug candidates and scaffolds that can target AMR. CPZEN-45 is under development as an anti-MDR/XDR-TB drug. The presence of an unpredictable pharmacophore, strong pharmacological activity, and diverse structures, makes these natural products highly attractive. The challenges associated with the discovery of drug targets and new scaffolds have been associated with the decline in research and development of antimicrobial drugs. However, we strongly believe that the use of new concepts in future will aid in the development of new search methods suitable for the identification of natural products that can function as antimicrobial agents.
In recent years, Europe and the United States have witnessed an inflow of several venture companies dealing with natural products. In addition, there has been a development of several new approaches using synthetic biology. The author wishes to contribute to the development of new-age natural products by the development of new approaches that will facilitate the exploration of the true potential of these gems hidden in nature.
References
Barriere SL. Clinical, economic and societal impact of antibiotic resistance. Expert Opin Pharmacother. 2015;16:151–3.
World Health Organization. World Health Day 2011: combat drug resistance: no action today means no cure tomorrow. World Health Organization; 2011. http://www.who.int/mediacentre/news/statements/2011/whd_20110407/en/.
O’Neill J. Antimicrobial resistance. Tackling a crisis for the health and wealth of nations. London: The Review on Antimicrobial Resistance; 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf#search=%273.+O’Neill+J%3A+Antimicrobial+resistance.%27.
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. London: The Review on Antimicrobial Resistance; 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
Ministry of foreign affairs of Japan. G7 Ise-Shima Vision for Global Health. 2016. https://www.mofa.go.jp/mofaj/files/000160273.pdf.
Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–61.
World Health Organization. Global tuberculosis report 2017. World Health Organization; 2017. http://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf.
Igarashi M, et al. Tubelactomicin A, a novel 16-membered lactone antibiotic, from Nocardia sp. I. Taxonomy, production, isolation and biological properties. J Antibiot. 2000;53:1096–101.
Igarashi M, et al. Tubelactomicin A, a novel 16-membered lactone antibiotic, from Nocardia sp. II. Structure elucidation. J Antibiot. 2000;53:1102–7.
Kinoshita N, et al. Nocardia vinacea sp. nov. Actinomycetologica. 2001;15:1–5.
Igarashi M, et al. Caprazamycin B, a novel anti-tuberculosis antibiotic, from Streptomyces sp. J Antibiot. 2003;56:580–3.
Igarashi M, et al. Caprazamycins, novel lipo-nucleoside antibiotics, from Streptomyces sp. II. Structure elucidation of caprazamycins. J Antibiot. 2005;58:327–37.
Igarashi M, et al. In: 42nd interscience, conference on antimicrobial agents and chemotherapy, September 22, Abstr. F-2031, San Diego: CA; 2002.
Ishizaki Y, et al. Inhibition of the first step in synthesis of the mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45. J Biol Chem. 2013;288:30309–19.
Isono K, et al. Liposidomycins: novel nucleoside antibiotics which inhibit bacterial peptidoglycan synthesis. J Antibiot. 1985;38:1617–21.
Kimura K, et al. New types of liposidomycins that inhibit bacterial peptidoglycan synthesis and are produced by Streptomyces. II. Isolation and structure elucidation. J Antibiot. 1998;51:647–54.
Yamaguchi H, et al. Capuramycin, a new nucleoside antibiotic. Taxonomy, fermentation, isolation and characterization. J Antibiot. 1986;39:1047–53.
Koga T, et al. Activity of capuramycin analogues against Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium intracellulare in vitro and in vivo. J Antimicrob Chemother. 2004;54:755–60.
Xie Y, et al. A new nucleosidyl-peptide antibiotic, sansanmycin. J Antibiot. 2007;60:158–61.
Takahashi Y, et al. Novel semisynthetic antibiotics from caprazamycins A-G: caprazene derivatives and their antibacterial activity. J Antibiot. 2013;66:171–8.
Takahashi Y, Igarashi M, Okada M. Anti-XDR-TB, anti-MDR-TB drug, and combination anti-tuberculoses drug. US9040502 B2, Microbial Chemistry Foundation, Disease Research Institute; 2015.
Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–54.
Gotoh Y, et al. Two-component signal transduction as potential drug targets in pathogenic bacteria. Curr Opin Microbiol. 2010;13:232–9.
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2008;6:17–27.
Martin PK, Li T, Sun D, Biek DP, Schmid MB. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J Bacteriol. 1999;181:3666–73.
Hancock L, Perego M. Two-component signal transduction in Enterococcus faecalis. J Bacteriol. 2002;184:5819–25.
Schreiber M, Res I, Matter A. Protein kinases as antibacterial targets. Curr Opin Cell Biol. 2009;21:325–30.
Matsushita M, Janda KD. Histidine kinases as targets for new antimicrobial agents. Bioorg Med Chem. 2002;10:855–67.
Fabret C, Hoch JA. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J Bacteriol. 1998;180:6375–83.
Dubrac S, Bisicchia P, Devine KM, Msadek T. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol. 2008;70:1307–22.
Okada A, et al. Targeting two-component signal transduction: a novel drug discovery system. Methods Enzymol. 2007;422:386–95.
Okada A, et al. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J Antibiot. 2010;63:89–94.
Igarashi M, et al. Waldiomycin, a novel WalK-histidine kinase inhibitor from Streptomyces sp. MK844-mF10. J Antibiot. 2013;66:459–64.
Eguchi Y, et al. Angucycline antibiotic waldiomycin recognizes common structural motif conserved in bacterial histidine kinases. J Antibiot. 2017;70:251–8.
Watanabe T, et al. Isolation and characterization of signermycin B, an antibiotic that targets the dimerization domain of histidine kinase WalK. Antimicrob Agents Chemother. 2012;56:3657–63.
Phillips JW, et al. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem Biol. 2011;26:955–65.
Sawa R, et al. Amycolamicin: a novel broad-spectrum antibiotic inhibiting bacterial topoisomerase. Chemistry. 2012;18:15772–81.
Igarashi M, Ishizaki Y, Takahashi Y. New antituberculous drugs derived from natural products: current perspectives and issues in antituberculous drug development. J Antibiot. 2017;71:15–25.
Acknowledgements
I would like to thank the past and present members of my institute and the collaborators who conducted a substantial amount of the work described herein. I extend special thanks to Prof. M Shibasaki, Dr M Hamada, Dr T Miyake, and Dr Y Takahashi of the Institute of Microbial Chemistry (BIKAKEN) and Prof. R Utsumi (Kinki University), Dr N Doi (The Research Institute of Tuberculosis), and Prof. M Okada (National Hospital Organization Kinki-Chuo Chest Medical center). I am also grateful to Dr Y Ishizaki of the Institute of Microbial Chemistry (BIKAKEN) for graphical support. The research presented herein was supported in part by the Research and Development Program for New Bio-Industry Initiatives (2006–2010) from the Bio-Oriented Technology Research Advancement Institution (BRAIN). ng contribution to the study of antibiotics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Masayuki Igarashi was awarded the Sumiki-Umezawa Memorial Award 2018 from the Japan Antibiotics Research Association. This review article is based on his award-winning research.
This article is dedicated to Dr Kiyoshi Isono’s 88 years anniversary, and his long-standing contribution to the study of antibiotics.
Rights and permissions
About this article
Cite this article
Igarashi, M. New natural products to meet the antibiotic crisis: a personal journey. J Antibiot 72, 890–898 (2019). https://doi.org/10.1038/s41429-019-0224-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0224-6
This article is cited by
-
Antimicrobial activity of some celastroloids and their derivatives
Medicinal Chemistry Research (2022)
-
Streptomyces sp SM01 isolated from Indian soil produces a novel antibiotic picolinamycin effective against multi drug resistant bacterial strains
Scientific Reports (2020)
-
Liposidomycin, the first reported nucleoside antibiotic inhibitor of peptidoglycan biosynthesis translocase I: The discovery of liposidomycin and related compounds with a perspective on their application to new antibiotics
The Journal of Antibiotics (2019)