Abstract
New hydroquinone derivatives bearing a vinyl alkyne, pestalotioquinols A and B, were isolated from a fungal culture broth of Pestalotiopsis microspora. The structures of these novel compounds were determined by interpretation of spectroscopic data (1D/2D NMR, MS, and IR), and the absolute configuration of the stereogenic center of pestalotioquinol A was assigned using the modified Mosher’s method. Nerve growth factor-differentiated neuronal PC12 cells were pretreated with pestalotioquinols A and B and removed from the medium, and then treated with a generator of peroxynitrite (ONOO–), a reactive nitrogen species, to induce cell death. The cytotoxicity of the treated cells was assessed by measuring lactate dehydrogenase leakage. As a result, 1–3 μM pretreatment of pestalotioquinols A and B rescued neuronal PC12 cells from peroxynitrite-induced cytotoxicity and the protective activity was sustained after removing each compound from the medium. These results demonstrate that pestalotioquinol derivatives are a new class of hydroquinones possessing a vinyl alkyne and exhibiting relatively high neuroprotective effects.
Similar content being viewed by others
Introduction
Peroxynitrite (ONOO–) is a strong reactive nitrogen species produced by the reaction of nitric oxide (•NO) and superoxide anion (•O2–). Its high reactivity allows peroxynitrite to oxidize a number of various cellular components such as lipids, proteins and DNA, and stimulate cell death by a variety of mechanisms. Peroxynitrite generation could be the cause of a number of pathological conditions ranging from atherosclerosis to inflammatory, autoimmune, heart and neurodegenerative diseases [1]. Protective agents against peroxynitrite-induced cell injury would lead to the treatment of neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease [2, 3].
In the past, we have focused on metabolites of fungi isolated from sands, soils, seaweed, mosses and other plants to obtain bioactive natural products. Extracts of fungal culture broth were purified by column chromatography to construct a chemical library, and a library screening resulted in the discovery of several bioactive compounds [4,5,6]. Of these compounds, neoechinulin A isolated from Eurotium rubrum Hiji025 was found to protect neuronal PC12 cells from cytotoxicity induced by the peroxynitrite generator 3-morpholinosydnonimine (SIN-1) [7, 8]. In addition, neoechinulin A improves memory functions in lipopolysaccharide-treated mice, and also exerts antidepressant-like effects [9]. Interestingly, neoechinulin A preferentially confers neuroprotection against nitrosative stresses caused by NO or NO-derived reactive species (i.e., SIN-1), but not against oxidative stresses (i.e., H2O2), and the neuroprotective activity of neoechinulin A was sustained after removing the compound from the medium [7, 10]. Although neoechinulin A possessed unique properties, a high concentration of neoechinulin A was required to exhibit the protective activity. Herein, we aimed to discover compounds with high neuroprotective activity from metabolites of fungi. In this screening program, neuronal PC12 cells were pretreated with compounds, removed from the medium, and then treated with SIN-1 in the absence of compounds to exclude antioxidants or direct scavengers of peroxynitrite as hits for neuroprotective compounds. The screening using the library containing fractions partially purified from the culture broths of fungi demonstrated that fractions of the culture extract of P. microspora rendered PC12 cells resistant to SIN-1-induced cytotoxicity (Figure S1). Activity-guided purification led to the discovery of novel hydroquinone derivatives designated pestalotioquinols A (1) and B (2) (Fig. 1). We herein report the isolation, structural elucidation, and neuroprotective effects of compounds 1 and 2.
Results and discussion
Repeated separations of a culture extract from P. microspora using silica gel yielded compounds 1 and 2. The molecular formula of C21H28O4 for compound 1 was determined by HRMS (FAB). The IR spectrum showed a band typical of a hydroxy group (3344 cm−1) and a weak band typical of an alkyne group (2196 cm−1). As shown in Table 1, the 13C NMR and DEPT spectroscopic data suggested the presence of eight quaternary carbons, five methine carbons, five methylene carbons and three methyl carbons. The 1H and 13C NMR and HMBC spectra established the presence of a monosubstituted hydroquinone, as shown in Table 1 and Fig. 2. HMBC correlations from protons of two terminal methyl groups (Me-11′) to oxygenated carbons C-10′ (δ 78.4) and C-11′ (δ 73.5), and consecutive 1H-1H COSY correlations from H-8′ to H-10′ revealed a partial structure of CH3-C(CH3)(OH)-CH(OH)-CH2-CH2-. The partial structure was found to be connected to an isoprene unit on the basis of 1H-1H COSY correlations and HMBC correlations from H-13′ to C-8′ and two olefinic carbons C-6′ (δ 123.9) and C-7′ (δ 135.8). HMBC correlations from H-3 to C-1′ (δ 83.7), and from exomethylene protons of H-12′ to C-3′ and C-2′ (δ 96.1) suggested the presence of a vinyl-acetylene moiety that was connected to the monosubstituted hydroquinone. The E-geometry of the C6′ = C7′ double bond was confirmed by NOESY correlations between H-5′ and H-13′, and H-6′ and H-8′ (Fig. 2). Thus, the structure of compound 1 was determined (Fig. 1), and it was named pestalotioquinol A.
To determine the absolute configuration at C-10′, Mosher’s (MTPA) ester of 1 was synthesized (Scheme 1). Compound 1 was reacted with methyl iodide in the presence of K2CO3 to give dimethyl ether 3. Esterification of 3 was performed with (R)- or (S)-MTPA chlorides, affording (S)- or (R)-MTPA ester 4 and 5. Δδ values (δ(S)–δ (R)) indicated that the absolute configuration at C-10′ was S (Fig. 3).
Compound 2 was found to have the same molecular formula of C21H28O4 as compound 1 by HRMS (FAB). As shown in Table 1, the 1H and 13C NMR spectra suggested that the structure of compound 2 was similar to compound 1, except for their side chain from C-6′ to C-11′. HMBC correlations from protons of two terminal methyl groups to olefinic carbons C-10′ (δ 124.3) and C-11′ (δ 132.3), and consecutive 1H-1H COSY correlations from H-8′ to H-10′ established an isoprene unit (Fig. 2). Two olefinic carbon signals of C-6′ and C-7′ in compound 1 was revealed to be replaced by those of C-6′ (δ 76.9) and C-7′ (δ 75.0) in compound 2 on the basis of HMBC correlations from H-13′ to C-6′ and C-7′, which suggested the presence of a diol at this position in agreement with the HRMS results. Thus, the structure of compound 2 (pestalotioquinol B) was elucidated (Fig. 1) and was further confirmed by 1H-1H COSY, HMQC and HMBC experiments.
Several hydroquinone derivatives bearing a vinyl acetylene moiety have been isolated from fungi such as Helminthosporium siccans, Phomopsis foeniculi and Sterium frustulosum [11,12,13,14]. Siccayne is one of the most studied antibiotic hydroquinone derivatives with an isopentenyne side chain (Fig. 1) [11, 15,16,17]. Foeniculoxin is a closely related derivative of pestalotioquinols A and B [13]. This compound is a phytotoxic metabolite produced by P. foeniculi and possesses a geranyl side chain (Fig. 1). Pestalotioquinols A and B are the first hydroquinone derivatives with a vinyl alkyne that possesses a side chain composed of three isoprene units.
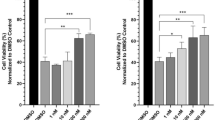
Pestalotioquinols A (1) and B (2) were evaluated for their neuroprotective effects on NGF-differentiated neuronal PC12 according to a procedure described previously [7]. In this assay, neuronal PC12 was treated with compounds 1 and 2, and then the compounds were removed from the medium. The pretreated cells were incubated with the peroxynitrite generator SIN-1 in the absence of the compounds, and the level of cytotoxicity was then assessed by measuring the leakage of lactate dehydrogenase (LDH) (Fig. 4a, b). As a result, both compounds were found to render PC12 cells resistant to SIN-1. Pretreatment with 1 μM of compounds 1 and 2 decreased the cytotoxicity of PC12 cells induced by 0.5 mM SIN-1. Morphologies of neuron-like PC12 treated with compounds 1 and 2 were confirmed by a microscope (Fig. 4c). Compound 1 showed a stronger neuroprotective effect than neoechinulin A, a fungus-derived neuroprotective compound reported previously (Fig. 4d) [7, 8].
Neuroprotective effects of compounds 1–3. PC12 cells were differentiated by NGF for 3 days. Differentiated PC12 cells were pretreated with the indicated concentration of compounds 1, 2 and 3 for 24 h. After removal of the compounds, cells were treated with 0.5 or 1.0 mM SIN-1 for 24 h. Cytotoxicity was evaluated by measuring released LDH. The values shown are the mean ± intra-assay deviation expressed as the S.D. a and b Neuroprotective effect of pestalotioquinols A (1) and B (2). c Photomicrographs of control cells, 0.5 mM SIN-1-treated cells, and 0.5 mM SIN-1+ 3 μM compound 1 or compound 2-treated cells. pestalotioquinols B (2). d Comparison of neuroprotective effects between pestalotioquinols A (1) and neoechinulin A (NeoA). e Neuroprotective effect of dimethyl ether of pestalotioquinol A (3)
The hydroquinone moiety is known to have antioxidant properties, and several neuroprotective natural products containing a hydroquinone moiety have been reported [18, 19]. To examine the importance of the hydroquinone moiety of the pestalotioquinols for neuroprotection, we compared the protective effects afforded by pestalotioquinol A (1) and dimethyl ether 3 (Fig. 4a, e). Although 10 μM of compound 3 decreased SIN-1-mediated cell death, the protective activity was weaker than that of 1, which suggested the hydroquinone of 1 is likely important for its neuroprotective activity.
In conclusion, two new hydroquinone derivatives bearing a vinyl alkyne moiety, pestalotioquinols A and B, were isolated from a fungal culture broth of P. microspora. To our knowledge, there are no reports on hydroquinone derivatives with a vinyl alkyne that possesses a side chain composed of three isoprene units. Pretreatment with pestalotioquinols A and B protected neuronal PC12 cells from peroxynitrite-induced cell death, and the protective activity was sustained after removing the compounds. In addition, our data indicated that the hydroquinone moiety of 1 is important for neuroprotective effects. Although a further mechanistic study of pestalotioquinols is required, the compounds may be developed as agents for treatment of reactive nitrogen species-related disorders such as Parkinson’s disease and Alzheimer’s disease in the future.
Materials and methods
General experimental procedure
Optical rotations were recorded on a JASCO P-2200 digital polarimeter (Jasco Corp., Tokyo, Japan) at room temperature. UV spectra were obtained on a UVmini-1240 spectrophotometer (Shimadzu corp., Kyoto, Japan). Infrared spectra (IR) were recorded on a JASCO FT/IR-4600 spectrophotometer (Jasco Corp.) and reported as wavenumbers (cm−1). 1H and 13C NMR spectra were recorded on a Bruker 400 MHz spectrometer (Avance DRX-400; Bruker, Billerica, MA), using CDCl3 solution (with TMS for 1H NMR and CDCl3 for 13C NMR as an internal reference). Chemical shifts are expressed in δ (ppm) relative to TMS or residual solvent resonance, and coupling constants (J) are expressed in Hz. Mass spectra (MS) were obtained on a JEOL mass spectrometer (JMS-700; JEOL, Tokyo, Japan). Analytical TLC was carried out on precoated silica gel 60 F254 plates (Merck, Darmstadt, Germany). Silica gel 60 N (Kanto Chemical, Tokyo, Japan) was used for silica gel column chromatography.
Preparation of the fraction library of fungal metabolites
Leaves, mosses, soils and sands were collected in various areas in Japan and suspended in sterilized water. The suspension was spread onto potato dextrose agar (PDA) plates (Difco & BBL, Flanklin Lakes, NJ, USA), and the plates were incubated for 1–2 weeks at 30 °C. Fungi growing on these plates were transferred onto individual PDA plates and cultured under the same conditions. Cultures were repeated 2–5 times to obtain pure mycelium strains. Each fungal stain isolated was cultured by transferring a small piece of agar from the culture plate into 2-l Erlenmeyer flasks containing potato dextrose broth (24 g) (Difco & BBL) in H2O (1.0 l). The culture was grown under static conditions at room temperature and in the dark for 3–6 weeks. The culture broth was filtered through cheesecloth to remove fungal mycelia and the filtrate was then extracted three times with 300 ml of CH2Cl2. The combined organic layer was evaporated in vacuo to obtain a crude extract. The crude extract was separated by silica gel column chromatography with CHCl3-MeOH (99:1–90:10) to produce several fractions to construct a fraction library. The library containing approximately 700 fractions obtained from more than 100 fungi was used to measure neuroprotective activity.
Isolation and of the fungus that produced the pestalotioquinols
The fungus that produced the pestalotioquinols reported here was isolated from leaves collected in Sagamihara, Kanagawa, Japan, and identified as P. microspora. The Internal Transcribed Spacer (ITS) region, which was amplified by PCR and sequenced, showed 100% sequence identity with P. microspora (GenBank accession number JN861776).
Extraction and purification of compounds
The fungal stain was cultured in 10 l of PDB and the culture was grown under static conditions at room temperature and in the dark for 21 days. The culture broth was then extracted using CH2Cl2, and the organic layer was evaporated in vacuo to obtain a crude extract (323 mg). This crude extract was separated by silica gel column chromatography (column size: 13 mmϕ, fraction volume: 7–10 ml) with CHCl3-MeOH (99:1–95:5, 250 ml) to give fractions 1–5. Fraction 3 was separated by silica gel column chromatography (column size: 13 mmϕ, fraction volume: 7–10 ml) with hexane-EtOAc (4:1-0:1, 200 ml) to give compound 1 (59.3 mg). Fraction 2 was separated by silica gel column chromatography (column size: 10 mmϕ, fraction volume: 4 ml) with hexane-EtOAc (4:1–0:1, 150 ml) to give fraction 2-1–2–7. Fraction 2-3 was separated by silica gel column chromatography (column size: 5 mmϕ, fraction volume: 2 ml) with toluene-EtOAc (1:1-0:1, 40 ml) to give compound 2 (2.5 mg).
Pestalotioquinol A (1): Yellow oil; \([{\rm{\alpha}}]^{22}_{\mathrm{D}}\) –10.8 (c 1.94, CHCl3); UV λMeOH max nm (ε) 264 (13,900), 276 (13,500), 328 (8,400); IR νmax (film) cm−1 3344, 2977, 2196, 1617, 1495; HRMS (FAB) m/z 367.1884 [M + Na]+ (calcd for C21H28O4Na, 367.1885); 13C and 1H data, see Table 1.
Pestalotioquinol B (2): Yellow oil; \([{\rm{\alpha}}]^{22}_{\mathrm{D}}\) –7.6 (c 0.125, CHCl3); UV λMeOH max nm (ε) 265 (9,500), 275 (9,300) 327 (5,400); IR νmax (film) cm−1 3394, 2977, 2196, 1606, 1495; HRMS (FAB) m/z 343.1908 [M–H]– (calcd for C21H27O4, 343.1909); 13C and 1H data, see Table 1.
Methylation of pestalotioquinol A
K2CO3 (14.7 mg) and MeI (0.2 ml) were added to a solution of pestalotioquinol A (12.2 mg) in acetone (0.5 ml), and the mixture was stirred at room temperature for 48 h. The reaction mixture was diluted with EtOAc (20 ml) and washed with H2O (3 × 10 ml). The crude product was purified by HPLC (PEGASIL ODS SP100, Senshu, Tokyo, Japan, 10 × 150 mm; solvent 50–100% MeOH, flow rate 3.0 ml min−1) to afford dimethylated product 3 (6.8 mg, 52%).
\([{\rm{\alpha}}]^{21}_{\mathrm{D}}\) –8.7 (c 0.275, CHCl3); UV \(\lambda^{\mathrm{MeOH}}_{\max}\) nm (ε) 240 (9,900), 267 (10,500), 325 (7,100); IR νmax (film) cm−1 3431, 3001, 2977, 2196, 1640, 1499, 1227; HRMS (FAB) m/z 395.2196 [M + Na]+ (calcd for C23H32O4Na, 395.2198); 1H NMR (400 MHz, CDCl3) δ 6.95 (1 H, d, J = 2.8 Hz), 6.84 (1 H, dd, J = 2.9, 9.0 Hz), 6.80 (1 H, d, J = 9.0 Hz), 5.44 (1 H, m), 5.30 (1 H, d, J = 1.7 Hz), 5.25 (1 H, m), 3.84 (3 H, s), 3.77 (3 H, s), 3.36 (1 H, m), 2.37-2.21 (4 H, m), 2.14-2.06 (2 H, m), 1.67 (3 H, s), 1.63-1.56 (1 H, m), 1.46-1.37 (1 H, m), 1.19 (3 H, s), 1.15 (3 H, s); 13C NMR (100 MHz, CDCl3) δ 154.4, 153.2, 135.7, 131.5, 124.2, 121.3, 118.1, 115.5, 113.0, 112.1, 93.8, 85.6, 78.1, 73.0, 56.5, 55.8, 37.4, 36.7, 29.5, 26.6, 26.4, 23.3, 15.9
Preparation of (R)- and (S)-MTPA esters of compound 3
Triethylamine (0.01 ml), DMAP (2.3 mg) and (R)-MTPACl (0.01 ml) were added to a solution of compound 3 (2.0 mg) in CH2Cl2 (1.0 ml), and the mixture was stirred at room temperature for 6 h. The reaction mixture was diluted with EtOAc (20 ml) and washed with H2O (3 × 10 ml). The crude product was purified by HPLC (PEGASIL ODS SP100, Senshu, Tokyo, Japan, 10 × 150 mm; solvent 50–100% MeOH, flow rate 3.0 ml min−1) to afford (S)-MTPA ester (4a) (1.0 mg, 32%). (R)-MTPA ester (4b) was prepared in a similar way using (S)-MTPACl.
(S)-MTPA ester 4a
HRMS (FAB) m/z 588.2700 [M]+ (calcd for C33H39F3O6, 588.2699); 1H NMR (400 MHz, CDCl3) δ 7.63-7.58 (2 H, m, aromatic signals of MTPA), 7.42-7.40 (3 H, m, aromatic signals of MTPA), 6.95 (1 H, d, J = 2.7 Hz, H-3), 6.83 (1 H, dd, J = 2.7, 9.0 Hz, H-5), 6.79 (1 H, d, J = 9.0 Hz, H-6), 5.43 (1 H, d, J = 1.8 Hz, H-12′), 5.29 (1 H, s, H-12′), 5.09 (1 H, m, H-6′), 4.98 (1 H, dd, J = 2.0, 10.1 Hz, H-10′), 3.83 (3 H, s, OMe), 3.76 (3 H, s, OMe), 3.58 (3 H, s, OMe of MTPA), 2.30 (4 H, m, H-4′ and H-5′), 1.88 (2 H, t, J = 7.4 Hz, H-8′), 1.69 (2 H, m, H-9′), 1.57 (3 H, s, H-13′), 1.22 (3 H, s, Me-11′), 1.16 (3 H, s, Me-11′)
(R)-MTPA ester 4b
HRMS (FAB) m/z 588.2697 [M]+ (calcd for C33H39F3O6, 588.2699); 1H NMR (400 MHz, CDCl3) δ 7.62-7.58 (2 H, m, aromatic signals of MTPA), 7.42-7.40 (3 H, m, aromatic signals of MTPA), 6.95 (1 H, d, J = 2.8 Hz, H-3), 6.84 (1 H, dd, J = 2.8, 9.0 Hz, H-5), 6.80 (1 H, d, J = 9.0 Hz, H-6), 5.43 (1 H, s, H-12′), 5.30 (1 H, s, H-12′), 5.14 (1 H, m, H-6′), 5.00-4.97 (1 H, m, H-10′), 3.83 (3 H, s, OMe), 3.76 (3 H, s, OMe), 3.58 (3 H, s, OMe of MTPA), 2.30 (4 H, m, H-4′ and H-5′), 1.98 (2 H, m, H-8′), 1.69 (2 H, m, H-9′), 1.61 (3 H, s, H-13′), 1.17 (3 H, s, Me-11′), 1.13 (3 H, s, Me-11′)
Cell culture
PC12 cells, a rat pheochromocytoma cell line, were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA), 5% horse serum (HS), 1% penicillin streptomycin (PS) (Thermo Fisher Scientific) in a humidified atmosphere of 5% CO2 at 37 °C.
Determination of neuroprotective effect of compounds
PC12 cells were seeded into poly-d-lysine coated 96-well plates (greiner bio-one, Kremsmünster, Australia) at a density of 5 × 103 cells/well in DMEM containing 10% FBS, 5% HS and 1% PS. On day 2, PC12 cells were differentiated with RPMI 1640 medium (Sigma-Aldrich) containing 50 ng ml−1 nerve growth factor (NGF) (Alomone labs, Jerusalem, Israel), 10% FBS and 1% PS for 3 days. The differentiated cells were incubated with test compounds or their vehicle (0.1% DMSO) in RPMI 1640 medium containing 50 ng ml−1 NGF, 10% FBS and 1% PS for 24 h. After the removal of test compounds, the pretreated cells were incubated with 0.5 mM or 1 mM SIN-1 (Dojindo, Kumamoto, Japan) for 24 h. Cytotoxicity was measured using a cytotoxicity LDH assay kit-WST (Dojindo) according to the manufacturer’s protocol.
References
Ramdial K, Franco MC, Estevez AG. Cellular mechanisms of peroxynitrite-induced neuronal death. Brain Res Bull. 2017;133:4–11.
Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–88.
Obata T. Nitric oxide and MPP+-induced hydroxyl radical generation. J Neural Transm. 2006;113:1131–44.
Takemoto K, et al. Bioactive dihydronaphthoquinone derivatives from Fusarium solani. J Nat Prod. 2014;77:1992–6.
Matsunaga H, et al. Isolation and structure of vanitaracin A, a novel anti-hepatitis B virus compound from Talaromyces sp. Bioorg Med Chem Lett. 2015;25:4325–8.
Nishikori S, et al. Anti-hepatitis C virus natural product from a fungus, Penicillium herquei. J Nat Prod. 2016;79:442–6.
Maruyama K, et al. Protective properties of neoechinulin A against SIN-1-induced neuronal cell death. J Biochem. 2004;136:81–7.
Kuramochi K, et al. Synthesis of neoechinulin A and derivatives. Synthesis. 2008;23:3810–8.
Sasaki-Hamada S, et al. Neoechinulin A induced memory improvements and antidepressant-like effects in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:155–61.
Akashi S, et al. Neoechinulin a imparts resistance to acute nitrosative stress in PC12 cells: a potential link of an elevated cellular reserve capacity for pyridine nucleotide redox turnover with cytoprotection. Biol Pharm Bull. 2012;35:1105–17.
Kupka J, Anke T, Steglich W, Zechlin L. Antibiotics from basidiomycetes. XI. The biological activity of siccayne, isolated from the marine fungus Halocyphina villosa J. & E. Kohlmeyer. J Antibiot. 1981;34:298–304.
Nair MSR, Anchel M. Frustulosinol, an antibiotic metabolite of Stereum frustulosum: revised structure of frustulosin. Phytochemistry. 1977;16:390–2.
Evidente A, et al. Foeniculoxin, a new phytotoxic geranylhydroquinone from Phomopsis foeniculi. Tetrahedron. 1994;50:10371–8.
Dubin GM, Fkyerat A, Tabacchi R. Acetylenic aromatic compounds from Stereum hirsutum. Phytochemistry. 2000;53:571–4.
Kim JH, Mahoney N, Chan KL, Molyneux RJ, Campbell BC. Secondary metabolites of the grapevine pathogen Eutypa Lata inhibit mitochondrial respiration, based on a model bioassay using the yeast Saccharomyces cerevisiae. Curr Microbiol. 2004;49:282–7.
Guerrero-Vasquez GA, Chinchilla N, Molinillo JM, Macias FA. Synthesis of bioactive speciosins G and P from Hexagonia speciosa. J Nat Prod. 2014;77:2029–36.
Pan Y, et al. Characterization of a prenyltransferase for iso-A82775C biosynthesis and generation of new congeners of chloropestolides. ACS Chem Biol. 2018;13:703–11.
Sasaki S, et al. Strongylophorine-8, a pro-electrophilic compound from the marine sponge Petrosia (Strongylophora) corticata, provides neuroprotection through Nrf2/ARE pathway. Biochem Biophys Res Commun. 2011;415:6–10.
Wei H, et al. (2S)-5, 2′, 5′-trihydroxy-7-methoxyflavanone, a natural product from Abacopteris penangiana, presents neuroprotective effects in vitro and in vivo. Neurochem Res. 2013;38:1686–94.
Acknowledgements
This work was supported by Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Private University Research Branding Project, 2016–2020, grants-in-aid from Japan Society for the Promotion of Science (KAKENHI 18K05343), and Program for Basic and Clinical Research on Hepatitis (18fk0210036j0001) from the Japan Agency for Medical Research and Development, AMED.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kanno, K., Tsurukawa, Y., Kamisuki, S. et al. Novel neuroprotective hydroquinones with a vinyl alkyne from the fungus, Pestalotiopsis microspora. J Antibiot 72, 793–799 (2019). https://doi.org/10.1038/s41429-019-0213-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-019-0213-9
This article is cited by
-
Recent advances on Pestalotiopsis genus: chemistry, biological activities, structure–activity relationship, and biosynthesis
Archives of Pharmacal Research (2023)
-
Determining the absolute configuration of vanitaracin A, an anti-hepatitis B virus agent
The Journal of Antibiotics (2022)