Abstract
Organocatalyzed asymmetric Friedel–Craft reactions have enabled the rapid construction of chiral molecules with highly enantioselectivity enriching the toolbox of chemists for producing complex substances. Here, we report N-heterocyclic carbene-catalyzed asymmetric indole Friedel–Crafts alkylation-annulation with α,β-unsaturated acyl azolium as the key intermediate, affording a large variety of indole-fused polycyclic alkaloids with excellent diastereo- and enantioselectivities. The reaction mechanism is also investigated, and the reaction products can be easily converted to highly functionalized indole frameworks with different core structures.
Similar content being viewed by others
Introduction
Indole-fused polycyclic scaffolds are ubiquitous in a large number of bioactive molecules and pharmacuticals, such as paxilline (potassium channel blocker), fischerindole L (antifungal activity), yuehchukene (strong anti-implantation activity), pergolide (medicine in the treatment of Parkinson’s disease), and hapalindole G (antimycotic activities) (Fig. 1)1,2,3,4. However, multiple steps have been used to make the key skeletons of these molecules, thus resulting in relatively low efficiency and atom economy5,6,7. Therefore, developing a protocol that can rapidly construct these polycyclic indole units is still highly desirable.
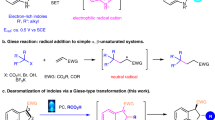
Asymmetric indole functionalization has been a field of intense focus in the recent decade. Friedel–Crafts alkylation of indoles using enones/enals has been a powerful strategy to achieve the above purpose8,9,10,11,12. Carbonyl activation of enones by Lewis/Brønsted acids and activation of enals via iminiums are prevalent activation modes (Fig. 2a)8,9,10,11,12. However, exploiting new activation modes of enones/enals holds great importance to further promote the influence of this strategy. On the other side, N-heterocyclic carbene (NHC)-catalyzed reactions13,14,15,16,17,18,19,20,21,22,23,24,25 mediated by α,β-unsaturated acyl azoliums19,26,27,28,29 have attracted increasing research interests owing to the great value in chiral cyclic molecule synthesis. Annulations of a series of carbon-based nucleophiles30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 with α,β-unsaturated acyl azoliums have been reported by Studer, Lupton, Bode, Enders, Chi, Ye, You, Du, Hui, Biju, and other groups. Additionally, the use of heteroatoms (N or S) as nucleophiles to react with α,β-unsaturated acyl azoliums has also been disclosed52,53,54,55,56. Despite these elegant reports, using α,β-unsaturated acyl azolium as the basic enal activation mode to achieve indole Friedel–Crafts alkylation remains a significant challenge to date (Fig. 2a). The difficulties arise from: (1) according to Studer and Mayr’s study, such a reaction is unfavorable because the electrophilicity of α,β-unsaturated acyl azoliums is 103–106 lower than that of iminiums57; (2) the competitive aza-Michael addition52,53,54,56 and N-acylation58 reactions are easy to occur under basic conditions; (3) the difficulty in regenerating NHC catalyst. Recently, Studer et al. achieved the intramolecular dearomative indole acylation via NHC catalysis59.
Here we address these challenges by installing an enone unit into indoles to trigger the following annulation, which provides additional driving force for the Friedel–Crafts alkylation, and the protocol affords a series of indole-fused polycyclic alkaloids with excellent diastero- and enantioselectivities (Fig. 2b).
Results
Optimization of the reaction conditions
Readily available indole enone 1a and enal 2a were selected to test our hypothesis (Fig. 3). The reaction using catalyst A60,61,62 with Cs2CO3 in CH2Cl2 led to the desired product 3a with excellent 99% ee, but in only 15% yield, and 1a was mostly recovered, indicating the low reactivity of α,β-unsaturated acyl azolium towards 1a (Table 1, entry 1). Then we tested catalysts60,61,62 B, C, D, and E, but in all cases, trace amount of 3a was formed (Table 1, entry 2). Catalyst F produced 3a with slightly lower yield and ee (Table 1, entry 3), and catalysts G, H, and I retarded the reaction (Table 1, entry 4). To our delight, increasing the temperature to 40 °C enhanced the yield (Table 1, entry 5), but other bases, such as K2CO3, KOAc, KOtBu, and DBU led to inferior results (Table 1, entries 6–9). CHCl3 or DCE proved inefficient and THF and toluene impeded the reaction (Table 1, entries 10–13). Gratifyingly, using more oxidant and M.S. and higher temperature could further enhance the yield (Table 1, entry 14), and 3a could be finally obtained in an acceptable 76% yield without affecting the enantioselectivity (Table 1, entry 15).
Model system used for reaction optimization. Conditions used for optimization of the catalyst, base, solvent, and temperature can be found in Table 1
Scope of 1,2,3,4-tetrahydrocyclopenta[b]indole alkaloids
Having identified the optimal conditions, we then evaluated the generality and limitations of this protocol. We found that the reaction could tolerate the introduction of electron-withdrawing 4-Cl, 3-Cl, 4-F, 4-Br, and electron-donating 4-Me substituents into the phenyl rings of the enone units, delivering 3b–3f with excellent 95–99% ee (Fig. 4, 3b–3f). Then we tested aryl enals equipped with F, Cl, and Br groups at the phenyl rings, and they all worked well under the optimal conditions, releasing 3g–3j with excellent 95–99% ee (Fig. 4, 3g–3j). Furan-substituted enal was surveyed and 3k was formed smoothly in 70% yield with 97% ee (Fig. 4, 3k). Enals with electron-rich aryl groups showed low conversion, but adding a Cl group into the indole ring had little influence on the outcome, affording 3l with 99% ee (Fig. 4, 3l). Moreover, varying simultaneously the substituent patterns of both enones and enals proved possible, delivering 3m–3r with 91–99% ee (Fig. 4, 3m–3r). Furthermore, methyl enone also worked well, forming 3s with 99% ee (Fig. 4, 3s). Additionally, enone with a Cl atom at the indole moiety could also cyclize with 4-Cl-C6H4-substituted enal, affording 3t in 67% yield with 99% ee (Fig. 4, 3t). Finally, when three substituted groups were introduced into the phenyl ring of enal, indole, and ketone unit, respectively, product 3u was also liberated with excellent 97% ee (Fig. 4, 3u). In all cases, the products were obtained as single diastereoisomers, and the absolute configuration of 3b was determined by the single crystal X-ray structure analysis (Fig. 4).
Scope of 1,2,3,4-tetrahydrocyclopenta[b]indole alkaloids. Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), A (0.04 mmol), oxidant (0.3 mmol), Cs2CO3 (0.3 mmol), CH2Cl2 (2 mL), 4 Å M.S. (800 mg), under argon atmosphere, 50 °C. All yields were isolated yields based on 1. All ee values were determined via HPLC analysis on a chiral stationary phase
Scope of 1,3,4,5-tetrahydrobenzo[cd]indole alkaloids
Next we investigated the possibility of making 1,3,4,5-tetrahydrobenzo[cd]indoles, which are also key units in many bioactive molecules. Indole enone 4a could react with 2a smoothly under slightly modified conditions, releasing 5a in 74% yield with excellent 99% ee (Fig. 5, 5a; Cs2CO3 led to 55% yield). Then we surveyed a series of enals bearing substituents with different electronic properties, and in all cases, the corresponding products were formed with excellent ee (Fig. 5, 5b–5e). Furan-substituted enal also worked well, affording 5f in 99% ee (Fig. 5, 5f). Aliphatic methyl enal proved amenable and 5g was formed with 85% ee in 64% yield (Fig. 5, 5g). Moreover, the reaction tolerated a variety of enones with both electron-poor and electron-rich aryl substituents, allowing access to 5h–5n with 90–95% ee (Fig. 5, 5h–5n). The reactions using thiophene or methyl-substituted enones proved successful, delivering 5o and 5p with excellent 93% and 97% ee, respectively (Fig. 5, 5o and 5p). Finally, we investigated the simultaneous variations of both enal and enone substituents, and different combination modes, such as aryl/aryl, aryl/alkyl, and alkyl/alkyl were all tolerated, and good to excellent ee values of the products were observed (Fig. 5, 5q–5v). The absolute configuration of 5a was determined by the single crystal X-ray structure analysis (Fig. 5)17.
Scope of 1,3,4,5-tetrahydrobenzo[cd]indole alkaloids. Reaction conditions: 4 (0.2 mmol), 2 (0.3 mmol), A (0.04 mmol), oxidant (0.3 mmol), Na2CO3 (0.3 mmol), CH2Cl2 (2 mL), 4 Å M.S. (800 mg), under argon atmosphere, 50 °C. All yields were isolated yields based on 4. All ee values were determined via HPLC analysis on a chiral stationary phase
Scale-up synthesis and product derivatizations
Scale-up synthesis of 3a and 5a proved possible, with no erosion of the ee detected (Fig. 6a, b). Furthermore, various simple reactions can be used to afford amide 6a, ester 6b/7a, and aldehyde 7b (Fig. 6c, d). The newly formed functional groups in these compounds can be readily used for further transformations.
Mechanistic studies
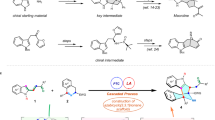
A series of experiments were conducted to gain more mechanistic insights (Fig. 7). The reaction using N-methyl indole enone 1a′ or 4a′ failed to deliver any products (Fig. 7a), indicating the vital role of indole N–H in the reaction. However, both Cs2CO3 and Na2CO3 cannot deprotonate indole N–H, showing that the nitrogen anion is not involved in the reaction (Fig. 7b). With catalytic amount of base or even without base, the reaction can still occur, albeit in lower yields (Fig. 7c). Using anion substrate 4aa we got N-acylation product 8a in 51% yield, and no annulation product was detected (Fig. 7d), which further excluded the existence of nitrogen anion in the catalytic cycle. Then, N-deuterium 4ab and 3-deuterium 4ac were used, but the reaction only led to 5a, indicating that proton transfer from indole to enal-derived intermediates may not occur (Fig. 7e). Furthermore, the reaction using deuterium enal 2a-d1 resulted in no erosion of D atom in the product, but the reaction was obvioulsy sluggish, and N-acylation product was also formed (Fig. 7f). However, using 2a-d3 we observed apparent D/H exchange during the reaction and only 63% deuterium was kept at the α-position of the ester moiety, indicating the probable equilibrium between azolium enolate and acyl azolium intermediates. Using 4m as the reference, we could estimate the KIE of the reaction to be 2.0, indicating that indole 3-position addition/C–H bond cleavage (from I to II) is possibly involved in the rate-determing step (RDS) (Fig. 7g).
Mechanistic studies and proposed mechanism. a Reactions using N-methyl-substituted indole enones. b Reactions in the absence of catalyst. c Reactions of 4a with 2a with catalystic amount of base or without base. d Reaction of indole anion. e Reactions of deuterium-indole substrates. f Reactions of 4a with deuterium-enals. g Evaluation of the KIE of the reaction. h Proposed mechanism
A plausible mechanism was proposed in Fig. 7h. The reaction of enal and NHC under oxidative conditions affords unsaturated acyl azolium I. The Friedel–Crafts reaction of indole enone and I results in enolate II (see the Supplementary Material for more details of this step); II can be protonated to produce III, and the process is reversible. Then the Michael addition happens to form a new enolate IV, and after lactonization, product 3a is produced, together with the regeneration of free carbene. Similar process happens when enone 4a is used, producing 5a as the final product.
Discussion
In summary, we have disclosed the NHC-catalyzed asymmetric indole Friedel–Crafts alkylation-annulation using α,β-unsaturated acyl azoliums for the first time, and the protocol afforded a large variety of indole-fused polycyclic alkaloids with excellent diastereo- and enantioselectivities. The competitive side reactions were suppressed, and mechanistic studies revealed that indole 3-position C–H bond cleavage is involved in the rate-determing step, and rapid equilibrium between azolium enolate and acyl azolium exists. This work further expands the application of NHC catalysis, and is also a valuable addition to the field of indole Friedel–Crafts alkylation. Further studies on NHC-catalyzed annulation reactions are ongoing in our group.
Methods
Genearl procedure for the annulation reaction of 1 and 2
Substrate 1a (247.10 mg 1.00 mmol), oxidant (612.45 mg, 1.50 mmol), 4 Å M.S. (2.00 g), Cs2CO3 (488.69 mg, 1.50 mmol) and catalyst (83.84 mg, 0.20 mmol) were added to CH2Cl2 (5 mL) at room temperature under argon atmosphere. After 15 min, 2a (198.09 mg, 1.50 mmol) was added into the reaction system. The reaction was stirred at 50 °C for 20 h. After that the residue was filtered on celite, the solvent was removed under reduced pressure and the residue was purified by flash chromatography with petroleum ether/ethyl acetate (v:v = 10:1) as eluent to afford the product 3a (271.5 mg, 72% yield).
General procedure for the annulation reaction of 4 and 2
Substrate 4a (247.10 mg 1.00 mmol), oxidant (612.45 mg, 1.50 mmol), 4 Å M.S. (3.00 g), Na2CO3 (159.00 mg, 1.50 mmol) and catalyst (83.84 mg, 0.20 mmol) were added to CH2Cl2 (5 mL) at room temperature under argon atmosphere. After 15 min, 2a (198.08 mg, 1.50 mmol) was added into the reaction system. The reaction was stirred at 50 °C for 20 h. After that the residue was filtered on celite, the solvent was removed under reduced pressure and the residue was purified by flash chromatography with petroleum ether/ethyl acetate (v:v = 10:1) as eluent to afford the product 5a (279.0 mg, 74% yield).
Typical procedure for the preparation of substrates
See Supplementary methods for more details.
Procedure for the product derivatizations
See Supplementary methods for more details.
Deuterium experiments
Proposed indole Friedel–Crafts alkylation mechanism
See Supplementary Fig. 7.
1H NMR and 13C NMR spectra of substrates and products
HPLC spectra of products
See Supplementary Figs. 80–126.
Crystallography
The CIF files for compounds 3b and 5a are available in Supplementary Data 1 and 2. Crystal data and structure refinement are shown in Supplementary Tables 1 and 2.
Data availability
Data for the crystal structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under the deposition numbers CCDC 1879978 (3b) and CCDC 1879979 (5a). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study, including compound characterization, are available within the paper and its Supplementary Information files, or from the corresponding authors on request.
References
Kong, Y.-C., Cheng, K.-F., Cambie, R. C. & Waterman, P. G. Yuehchukene: a novel indole alkaloid with anti-implantation activity. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39850000047 (1985).
Moore, R. E., Cheuk, C., Yang, X.-Q. G. & Patterson, G. M. L. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 52, 1036–1043 (1987).
Park, A., Moore, R. E. & Patterson, G. M. L. Fischerindole L, a new isonitrile from the terrestrial blue-green alga fischerella muscicola. Tetrahedron Lett. 33, 3257–3260 (1992).
Sanchez, M. & McManus, O. B. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35, 963–968 (1996).
Sheu, J.-H., Chen, Y.-K. & Hong, Y.-L. V. An efficient synthesis of yuehchukene. Tetrahedron Lett. 32, 1045–1046 (1991).
Fukuyama, T. & Chen, X. Stereocontrolled synthesis of (–)-hapalindole G. J. Am. Chem. Soc. 116, 3125–3126 (1994).
Richter, J. M. et al. Enantiospecific total synthesis of the hapalindoles, fischerindoles, and welwitindolinones via a redox economic approach. J. Am. Chem. Soc. 130, 17938–17954 (2008).
Poulsen, T. B. & Jørgensen, K. A. Catalytic asymmetric Friedel–Crafts alkylation reactions-copper showed the way. Chem. Rev. 108, 2903–2915 (2008).
You, S.-L., Cai, Q. & Zeng, M. Chiral Brønsted acid catalyzed Friedel–Crafts alkylation reactions. Chem. Soc. Rev. 38, 2190–2201 (2009).
Zeng, M. & You, S.-L. Asymmetric Friedel-Crafts alkylation of indoles: the control of enantio- and regioselectivity. Synlett 2010, 1289–1301 (2010).
Bartoli, G., Bencivenni, G. & Dalpozzo, R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 39, 4449–4465 (2010).
Dalpozzo, R. Strategies for the asymmetric functionalization of indoles: an update. Chem. Soc. Rev. 44, 742–778 (2015).
Enders, D., Niemeier, O. & Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 107, 5606–5655 (2007).
Biju, A. T., Kuhl, N. & Glorius, F. Extending NHC-catalysis: coupling aldehydes with unconventional reaction partners. Acc. Chem. Res. 44, 1182–1195 (2011).
Grossmann, A. & Enders, D. N-Heterocyclic carbene catalyzed domino reactions. Angew. Chem. Int. Ed. 51, 314–325 (2012).
Bugaut, X. & Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 41, 3511–3522 (2012).
Bode, J. W. An internal affair. Nat. Chem. 5, 813–815 (2013).
Chen, X.-Y. & Ye, S. Enantioselective cycloaddition reactions of ketenes catalyzed by N-heterocyclic carbenes. Synlett 24, 1614–1622 (2013).
Ryan, S. J., Candish, L. & Lupton, D. W. Acyl anion free N-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 42, 4906–4917 (2013).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Flanigan, D. M., Romanov-Michailidis, F., White, N. A. & Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 115, 9307–9387 (2015).
Menon, R. S., Biju, A. T. & Nair, V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon–carbon bond-forming reactions. Chem. Soc. Rev. 44, 5040–5052 (2015).
Chen, X. Y., Liu, Q., Chauhan, P. & Enders, D. N-Heterocyclic carbene catalysis via azolium denolates: an efficient strategy for remote enantioselective functionalizations. Angew. Chem. Int. Ed. 57, 3862–3873 (2018).
Murauski, K. J. R., Jaworski, A. A. & Scheidt, K. A. A continuing challenge: N-heterocyclic carbene-catalyzed syntheses of γ-butyrolactones. Chem. Soc. Rev. 47, 1773–1782 (2018).
Chen, X.-Y., Li, S., Vetica, F., Kumar, M. & Enders, D. N-Heterocyclic-carbene-catalyzed domino reactions via two or more activation modes. iScience 2, 1–26 (2018).
De Sarkar, S., Biswas, A., Samanta, R. C. & Studer, A. Catalysis with N-heterocyclic carbenes under oxidative conditions. Chem. Eur. J. 19, 4664–4678 (2013).
Mahatthananchai, J. & Bode, J. W. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res. 47, 696–707 (2014).
Levens, A. & Lupton, D. W. All-carbon (4 + 2) annulations catalysed by N-heterocyclic carbenes. Synlett 28, 415–424 (2017).
Zhang, C., Hooper, J. F. & Lupton, D. W. N-Heterocyclic carbene catalysis via the α,β-unsaturated acyl azolium. ACS Catal. 7, 2583–2596 (2017).
Ryan, S. J., Candish, L. & Lupton, D. W. N-Heterocyclic carbene-catalyzed generation of α,β-unsaturated acyl imidazoliums: synthesis of dihydropyranones by their reaction with enolates. J. Am. Chem. Soc. 131, 14176–14177 (2009).
De Sarkar, S. & Studer, A. NHC-catalyzed Michael addition to α,β-unsaturated aldehydes by redox activation. Angew. Chem. Int. Ed. 49, 9266–9269 (2010).
Rong, Z.-Q., Jia, M.-Q. & You, S.-L. Enantioselective N-heterocyclic carbene-catalyzed Michael addition to α,β-unsaturated aldehydes by redox oxidation. Org. Lett. 13, 4080–4083 (2011).
Lu, Y., Tang, W., Zhang, Y., Du, D. & Lu, T. N-Heterocyclic carbene-catalyzed annulations of enals and ynals with indolin-3-ones: synthesis of 3,4-dihydropyrano[3,2-b]indol-2-ones. Adv. Synth. Catal. 355, 321–326 (2013).
Chen, X.-Y. et al. N-Heterocyclic carbene catalyzed cyclocondensation of α,β-unsaturated carboxylic acids: enantioselective synthesis of pyrrolidinone and dihydropyridinone derivatives. Angew. Chem. Int. Ed. 53, 11611–11615 (2014).
Perveen, S. et al. Enantioselective synthesis of 1,2-dihydronaphthalenes via oxidative N-heterocyclic carbene catalysis. Org. Lett. 19, 2470–2473 (2017).
Gillard, R. M., Fernando, J. E. M. & Lupton, D. W. Enantioselective N-heterocyclic carbene catalysis via the dienyl acyl azolium. Angew. Chem. Int. Ed. 57, 4712–4716 (2018).
Ryan, S. J., Candish, L. & Lupton, D. W. N-Heterocyclic carbene-catalyzed (4 + 2) cycloaddition/decarboxylation of silyl dienol ethers with α,β-unsaturated acid fluorides. J. Am. Chem. Soc. 133, 4694–4697 (2011).
Candish, L., Levens, A. & Lupton, D. W. Enantioselective all-carbon (4 + 2) annulation by N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 136, 14397–14400 (2014).
Yetra, S. R., Mondal, S., Mukherjee, S., Gonnade, R. G. & Biju, A. T. Enantioselective synthesis of spirocyclohexadienones by NHC-catalyzed formal [3 + 3] annulation reaction of enals. Angew. Chem. Int. Ed. 55, 268–272 (2016).
Zhang, G. et al. Enantioselective intermolecular all-carbon [4 + 2] annulation via N-heterocyclic carbene organocatalysis. Chem. Commun. 53, 13336–13339 (2017).
Kaeobamrung, J., Mahatthananchai, J., Zheng, P. & Bode, J. W. An enantioselective Claisen rearrangement catalyzed by N-heterocyclic carbenes. J. Am. Chem. Soc. 132, 8810–8812 (2010).
Li, G.-T., Li, Z.-K., Gu, Q. & You, S.-L. Asymmetric synthesis of 4-aryl-3,4-dihydrocoumarins by N-heterocyclic carbene catalyzed annulation of phenols with enals. Org. Lett. 19, 1318–1321 (2017).
Kravina, A. G., Mahatthananchai, J. & Bode, J. W. Enantioselective, NHC-catalyzed annulations of trisubstituted enals and cyclic N-sulfonylimines via α,β-unsaturated acyl azoliums. Angew. Chem. Int. Ed. 51, 9433–9436 (2012).
Cheng, J., Huang, Z. & Chi, Y. R. NHC organocatalytic formal LUMO activation of α,β-unsaturated esters for reaction with enamides. Angew. Chem. Int. Ed. 52, 8592–8596 (2013).
Bera, S., Samanta, R. C., Daniliuc, C. G. & Studer, A. Asymmetric synthesis of highly substituted β-lactones through oxidative carbene catalysis with LiCl as cooperative Lewis acid. Angew. Chem. Int. Ed. 53, 9622–9626 (2014).
Bera, S., Daniliuc, C. G. & Studer, A. Enantioselective synthesis of substituted δ-lactones by cooperative oxidative N-heterocyclic carbene and Lewis acid aatalysis. Org. Lett. 17, 4940–4943 (2015).
Liang, Z. Q., Wang, D. L., Zhang, H. M. & Ye, S. Enantioselective synthesis of bicyclic δ-lactones via N-heterocyclic carbene-catalyzed cascade reaction. Org. Lett. 17, 5140–5143 (2015).
Chen, X.-Y. et al. Highly enantioselective kinetic resolution of Michael adducts through N-heterocyclic carbene catalysis: an efficient asymmetric route to cyclohexenes. Chem. Eur. J. 24, 9735–9738 (2018).
Liu, Q., Chen, X.-Y., Puttreddy, R., Rissanen, K. & Enders, D. N-Heterocyclic carbene catalyzed quadruple domino reactions: asymmetric synthesis of cyclopenta[c]chromenones. Angew. Chem. Int. Ed. 57, 17100–17103 (2018).
Wang, J. et al. Carbene-catalyzed enantioselective addition of benzylic carbon to unsaturated acyl azolium for rapid synthesis of pyrrolo[3,2-c]quinolones. ACS Catal. 8, 9859–9864 (2018).
Mukherjee, S., Ghosh, A., Marelli, U. K. & Biju, A. T. N-Heterocyclic carbene-catalyzed Michael–Michael-lactonization cascade for the enantioselective synthesis of tricyclic δ-Lactones. Org. Lett. 20, 2952–2955 (2018).
Zhang, H.-R., Dong, Z.-W., Yang, Y.-J., Wang, P.-L. & Hui, X.-P. N-Heterocyclic carbene-catalyzed stereoselective cascade reaction: synthesis of functionalized tetrahydroquinolines. Org. Lett. 15, 4750–4753 (2013).
Wu, X. et al. Enantioselective nucleophilic β-carbon atom amination of enals: carbene-catalyzed formal [3 + 2] reactions. Angew. Chem. Int. Ed. 55, 12280–12284 (2016).
Wu, X. et al. Construction of fused pyrrolidines and β-lactones by carbene-catalyzed C–N, C–C, and C–O bond formations. Angew. Chem. Int. Ed. 56, 4201–4205 (2017).
Fang, C., Lu, T., Zhu, J., Sun, K. & Du, D. Formal [3 + 4] annulation of α,β-unsaturated acyl azoliums: access to enantioenriched N–H-free 1,5-benzothiazepines. Org. Lett. 19, 3470–3473 (2017).
Mukherjee, S., Shee, S., Poisson, T., Besset, T. & Biju, A. T. Enantioselective N-heterocyclic carbene-catalyzed cascade reaction for the synthesis of pyrroloquinolines via N–H functionalization of indoles. Org. Lett. 20, 6998–7002 (2018).
Samanta, R. C. et al. Nucleophilic addition of enols and enamines to α,β-unsaturated acyl azoliums: mechanistic studies. Angew. Chem. Int. Ed. 51, 5234–5238 (2012).
Ta, L. & Sundén, H. Oxidative organocatalytic chemoselective N-acylation of heterocycles with aromatic and conjugated aldehydes. Chem. Commun. 54, 531–534 (2018).
Bera, S., Daniliuc, C. G. & Studer, A. Oxidative N-heterocyclic carbene catalyzed dearomatization of indoles to spirocyclic indolenines with a quaternary carbon stereocenter. Angew. Chem. Int. Ed. 56, 7402–7406 (2017).
Kerr, M. S., Read de Alaniz, J. & Rovis, T. A highly enantioselective catalytic intramolecular Stetter reaction. J. Am. Chem. Soc. 124, 10298–10299 (2002).
Kerr, M. S. & Rovis, T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric Stetter reaction. J. Am. Chem. Soc. 126, 8876–8877 (2004).
Lv, H., Zhang, Y.-R., Huang, X.-L. & Ye, S. Asymmetric dimerization of disubstituted ketenes catalyzed by N-heterocyclic carbenes. Adv. Synth. Catal. 350, 2715–2718 (2008).
Acknowledgements
This work was supported by NSFC (21871260, 21502192, 21402199), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), Fujian Natural Science Foundation (2018J05035), and China Postdoctoral Science Foundation (2018M630734).
Author information
Authors and Affiliations
Contributions
X.F. designed the project. M.A. conducted most of the experiments. S.P., X.K. and S.T.Z. conducted part of the experiments. X.F. wrote the manuscript. M.A. and S.Y. prepared the Supplementary Material. J.L. tested the single crystal structures. W.X. and S.Y. contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anwar, M., Yang, S., Xu, W. et al. Carbene-catalyzed asymmetric Friedel–Crafts alkylation-annulation sequence and rapid synthesis of indole-fused polycyclic alkaloids. Commun Chem 2, 85 (2019). https://doi.org/10.1038/s42004-019-0188-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-019-0188-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.