Abstract
Over the past two decades, we have witnessed a strong interest in developing Mg3Sb2 and related CaAl2Si2-type materials for low- and intermediate-temperature thermoelectric applications. In this review, we discuss how computations coupled with experiments provide insights for understanding chemical bonding, electronic transport, point defects, thermal transport, and transport anisotropy in these materials. Based on the underlying insights, we examine design strategies to guide the further optimization and development of thermoelectric Mg3Sb2-based materials and their analogs. We begin with a general introduction of the Zintl concept for understanding bonding and properties and then reveal the breakdown of this concept in AMg2X2 with a nearly isotropic three-dimensional chemical bonding network. For electronic transport, we start from a simple yet powerful atomic orbital scheme of tuning orbital degeneracy for optimizing p-type electrical properties, then discuss the complex Fermi surface aided by high valley degeneracy, carrier pocket anisotropy, and light conductivity effective mass responsible for the exceptional n-type transport properties, and finally address the defect-controlled carrier density in relation to the electronegativity and bonding character. Regarding thermal transport, we discuss the insight into the origin of the intrinsically low lattice thermal conductivity in Mg3Sb2. Furthermore, the anisotropies in electronic and thermal transport properties are discussed in relation to crystal orbitals and chemical bonding. Finally, some specific challenges and perspectives on how to make further developments are presented.
Similar content being viewed by others
Introduction
The increasing energy consumption worldwide pushes significant efforts in developing alternative energy technologies. Thermoelectric (TE) technology, capable of converting waste heat into electrical energy, shows potential for waste heat-harvesting applications with a great advantage that TE devices are entirely in solid state with no moving parts, compact, quiet, and maintenance free.1,2 One key bottleneck of this promising technology is its low conversion efficiency, which is essentially limited by low-performing materials. The performance of a TE material can be characterized by the dimensionless figure of merit, zT = α2σT/(κL + κe), where α is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature, κL is the lattice thermal conductivity, and the electronic thermal conductivity κe is related to σ through the Wiedemann–Franz law κe = LσT (L denotes the Lorenz number). The interdependent correlation among the TE transport parameters invokes numerous efforts3,4,5,6,7,8,9,10 worldwide on decoupling these parameters so as to improve zT. In general, achieving a superior zT requires a combined effort of improving the power factor α2σ and reducing the lattice thermal conductivity.
AB2X2 compounds with the CaAl2Si2-type structure, usually described as Zintl phases, are well recognized due to their promising TE properties. In these compounds, A is an alkaline earth or a divalent rare earth element, B is a transition metal or a main group element, and X usually comes from group 14, 15, or occasionally group 13.11,12 The rich variety of compositions covered by AB2X2 (CaAl2Si2-type) compounds enables considerable chemical tunability of the electronic and thermal transport properties. During the past two decades, both theoretical and experimental aspects in CaAl2Si2-type TE materials have undergone a rapid development,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52 which results in a significant improvement of zT above unity (Fig. 1). Experimentally, strategies such as doping,45,46,47,49,50 alloying,15,16,18,19,21,23,25,29,35,36 and controlling vacancy concentrations27 have been used to optimize electrical transport properties and to reduce thermal conductivity through point defect scattering. As nearly all these compounds are persistently p-type, it is a remarkable breakthrough that the low-cost Te-doped Mg3Sb2-based compounds were recently discovered to show exceptional n-type TE properties at low and intermediate temperatures (Fig. 1),53,54,55 comparable or even superior to the commercial n-type TE materials, such as Bi2Te3 and PbTe. Hence, significant research efforts56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73 on this promising material system are currently ongoing. Theoretically, electronic structures,20,74,75,76,77 chemical bonding,11,12,78,79 defects,55,60,61,80 and phonon-related properties81,82,83,84 have been extensively studied to understand the transport properties. The interplay between theory and experiment has given rise to many important guidelines based on the underlying physical and chemical insights for optimizing CaAl2Si2-type TEs.
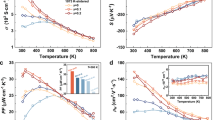
a, b Timelines of a the maximum zT and b the average zT values of the reported AB2X2 thermoelectric materials with the CaAl2Si2-type structure. The average zT is calculated by the direct integration method within the entire measurement temperature range. The experimental data are taken from refs. 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71
In this review, we summarize some of the successful guiding principles for understanding and rationalizing the electrical and thermal transport in Mg3Sb2 and its derivatives with the CaAl2Si2-type structure. We show how computational efforts integrated with experiments lead to additional physical and chemical insights for the profound understanding of chemical bonding, electronic transport, defect chemistry, phonon transport, and transport anisotropy. We start from general introductions of crystal structure and the Zintl concept that has been widely applied to understand the structure, bonding, and electronic transport in CaAl2Si2-type compounds. After that, we reveal the nearly isotropic three-dimensional (3D) chemical bonding network in AMg2X2, where the Zintl formalism is no longer applicable. For p-type electronic transport, we discuss how electronic transport can be optimized by minimizing the crystal orbital splitting energy via forming solid solutions and tuning biaxial strains, whereas for n-type transport we reveal the multi-valley conduction bands and complex Fermi surface as the electronic origin of the extraordinary n-type TE properties. Then we review the defect chemistry of the intrinsic p-type behavior and the surprising n-type behavior under different thermodynamic states, followed by highlighting the defect-controlled carrier transport and its correlation with the electronegativity and bonding character. For thermal transport, we review the studies on exploring the origin of the intrinsically low lattice thermal conductivity in Mg3Sb2 from first principles calculations. Moreover, we discuss the anisotropy in electrical and thermal transport properties with respect to crystal orbitals and chemical bonding. Finally, we conclude with some current challenges and prospects for future development.
Crystal structure
AB2X2 compounds with the trigonal CaAl2Si2-type structure85 (space group: P\(\bar 3\)m1) can be viewed as the Aδ+ cationic layers intercalated between the tightly bound [B2X2]δ− anionic layers (Fig. 2a). The three types of atoms A, B, and X occupy three different crystallographic sites 1a (0, 0, 0), 2d (1/3, 2/3, zB), and 2d (1/3, 2/3, zX) with point symmetries of \(\bar 3\)m, 3m, and 3m, respectively. In the [B2X2]δ− networks, B has a normal tetrahedral coordination, while X shows a unique distorted umbrella-like local environment.12 As a result, the vertical B-X bond (d3) along the c axis is usually longer than the three symmetry-equivalent tilted B-X bonds (d2). Taking into account the cationic Aδ+ layers, the interlayer A-X bonds (d1) hold together the alternating cationic and anionic layers. Unlike the B atoms being tetrahedrally coordinated by the X atoms, each A atom occupies the octahedral site with six equal adjacent bonds. Each X atom, surrounded by four B atoms and three A atoms, has seven adjacent bonds including one vertical B-X bond, three tilted B-X bonds, and three interlayer A-X bonds (Fig. 2c–e).
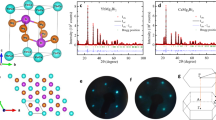
a Crystal structure of layered Zintl phases A(Zn,Cd)2Sb2 with the CaAl2Si2-type structure, showing a clear distinction between the ionic Aδ+ layer and the covalent [B2X2]δ− layer. b A more accurate structural view of AMg2X2 with the CaAl2Si2-type structure, which shows comparable interlayer and intralayer bonds being mostly ionic with partial covalent nature (high polarity). c–e Coordination environments of the three nonequivalent atoms c A, d B, and e X in AB2X2 compounds with the CaAl2Si2-type structure. f Static deformation electron density map on (110) plane of Mg3Sb2 with a nearly isotropic three-dimensional bonding network (\(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}} \approx {\mathrm{1}}\)). The contour interval is 0.006 e Å−3. Positive (negative) contours are plotted with red solid (blue dotted) lines. g Noncovalent interaction analysis with reduced density gradient as a function of sign(λ2)ρ for the interlayer and intralayer interactions in Mg3Sb2. sign(λ2) is the sign of the second eigenvalue of the electron density Hessian matrix.101 Figure is reproduced with permission from ref. 79, CC-BY-4.0
Mg3X2 (X = As, Sb, and Bi) crystallizing in the inverse α-La2O3 structure is a special case of the CaAl2Si2 (AB2X2) structure with A and B being, respectively, Mg1 and Mg2.86 The crystal structures of Mg3Sb2 and Mg3Bi2 were reported by Zintl and Husemann in 1933,87 much earlier than those of ternary derivatives. From the reported phase diagram,88,89 Mg3Sb2 and Mg3Bi2 show a phase transition from the low-temperature α phase to the high-temperature β phase at ~900 and ~700 °C, respectively. The high-temperature β phase is superionic with liquid-like magnesium ions and it is assumed to have a cubic structure,90 but the exact crystal structure remains unclear.
Zintl formalism
The Zintl concept91,92 plays a vital role in understanding structure, chemical bonding, and properties of a wide range of solid-state materials. Within this simple but powerful concept, the mostly ionic cations are considered merely as electron donors, donating their electrons to the covalently bonded anionic substructures. The covalent bonding in the anionic substructures ensures a significant orbital overlap, a light band mass, and thereby a high carrier mobility, whereas the ionic cations are suitable for doping with the aim to tune the carrier density without affecting the covalent anionic networks.93,94 Such bonding features, as well as ideal band gaps, make charge-balanced Zintl compounds very promising for TE applications.
As described by Hoffmann et al.,11,12 AB2X2 compounds with the CaAl2Si2-type structure can easily be understood as Zintl phases, where the covalently bonded [B2X2]δ− layers receive electrons from the ionic Aδ+ layers. Based on the aforementioned Zintl concept, considerable efforts have been made to explore AB2X2-type TE compounds and optimize them via doping, substituting, or creating vacancies on the cationic A sites. One notable example can be seen in Ca1−xYbxZn2Sb2 alloys reported by Gascoin et al.,15 which show the fine tunability of the carrier concentration through doping or substituting Ca with Yb. The alloying of Ca and Yb indeed has a negligible effect on the covalent anionic [Zn2Sb2]2− networks, so that the good carrier mobility and ideal energy gap are maintained. In addition, this alloying creates disorder in the cationic layers, which results in a reduction in thermal conductivity and an enhancement in zT.15 Ever since this early work, the Zintl formalism has been broadly applied in developing and optimizing TE AZn2Sb2, ACd2Sb2, and their alloys.20,23,27,32 In these compounds, however, it was found that the hole mobility may be greatly affected by changing the cation A from alkaline earth elements to rare earth elements.20 Different synthesis methods were also found to result in a great change in carrier mobility of AZn2Sb2.95 These results are likely induced by the change in carrier scattering time τ because the effective mass of the valence band is shown to be unaffected by varying the cation A.20
Nearly isotropic 3D bonding network in AMg2X2: Breakdown of the Zintl formalism
Despite being very successful in rationalizing the electronic transport, the Zintl formalism and electron counting are not universal and can only provide a qualitative insight. It does not give us any indications of the site preference, bond length difference in anionic networks, or the reason why intrinsic p-type Mg3Sb2 shows very poor carrier mobility. More accurate theoretical calculations based on quantum mechanics or density functional theory (DFT) are required to understand the structure and chemical bonding.
Using the crystal orbital overlap population approach based on the extended Hückel calculations, Hoffmann et al.12 gave a rational explanation for the unusual umbrella-like local coordination environment of the anionic X atom and the bond length difference in the [B2X2]δ− networks of the CaAl2Si2 (AB2X2) structure through making and breaking chemical bonds. In addition, they compared the local and extended constructions of the bonding in transition metal CaAl2Si2-type compounds with those in ThCr2Si2-type compounds and explained why compounds with the B-site elements being occupied by transition metals with the d0, d5, and d10 configurations only form the CaAl2Si2-type structure.11 Burdett and Miller also conducted extended Hückel calculations to reveal the formalism of the Al2Si2 fragment in the CaAl2Si2-type structure and extended the fragment formalism to several other main group compounds.77 The above studies were conducted only based on the chemical bonds in the [B2X2]δ− networks, neglecting the effect of the cationic Aδ+ layer. The contribution of the cationic Ca2+ layer to the chemical bonding and electronic structure of CaAl2Si2 was investigated by Alemany et al. using DFT calculations.95 They found that the cationic layers show an important contribution to the covalent bonding of CaAl2Si2, although the cationic layers do not play a dominant role in the electronic structure and the origin of the conductivity behavior. In addition to these significant studies11,12,78,96 on rationalizing structural formation and electronic structure using the molecular orbital approaches, Grin et al.18 studied the chemical bonding in YbCd2Sb2 using the electron localizability indicator and showed that four-center bonding exists in this compound and the whole bonding picture can be described by the closed-shell configuration [Yb2+][Cd2+]2[Sb3−]2. Based on the analysis of the electron density difference, Toberer et al.20 discussed the chemical bonding in AZn2Sb2 (A = Sr, Ca, and Yb) and revealed the largely covalent nature of the Zn-Sb bonds as well as the charge accumulation from Sb to A. The A-Sb bond was found to be very asymmetric and slightly larger in YbZn2Sb2 than in SrZn2Sb2 or CaZn2Sb2, which might be attributed to the less electron transfer from the cation A to the anionic Zn2Sb2 slabs induced by the larger electronegativity of Yb in comparison to Sr or Ca.20 Using DFT calculations, Li et al. revealed a much lower shear strength in Mg3Sb2 compared to CaMg2Sb2 and CaZn2Sb2, which was explained by the weaker interlayer Mg-Sb bond in Mg3Sb2.97 Despite significant efforts on understanding chemical bonding, most of these efforts are qualitative and there is clearly a lack of a quantitative chemical bonding description of CaAl2Si2-type compounds based on topological analysis of the electron density.
Recent theoretical calculations extend our knowledge of chemical bonding in CaAl2Si2-type compounds. Quantitative analysis of full DFT electron density by Zhang et al.79 elucidated that the Zintl formalism is perfectly applicable in AZn2Sb2 and ACd2Sb2 but not in AMg2X2 (including Mg3X2). The analysis was conducted using the quantum theory of atoms in molecules (QTAIM) developed by Bader.98 QTAIM is based on the analysis of critical points of the electron density ρ, which are the points satisfying ∇ρ = 0. The readers are referred to ref. 98 for the details of the method. The idea is clearly proved by the result of Bader atomic charges (see Table 1). In CaZn2Sb2 and CaCd2Sb2, the cationic and anionic layers show a remarkable difference in the degree of ionicity99 estimated from atomic charges, i.e., the cationic layers are largely ionic while the anionic layers are mostly covalent. In contrast, nearly complete charge transfers are observed for all atoms in AMg2X2, which elucidates the mostly ionic nature in both cationic and anionic layers. This indicates that any description of AMg2X2, especially Mg3Sb2, as layered Zintl phases in all previous literature is incorrect.
Chemical bonds in AB2X2 (CaAl2Si2-type) compounds can be described as polar bonds based on the topological properties of the bond critical points (BCPs) using the classification scheme by Gatti.100 The covalency of the intralayer bonds in the B2X2 networks, however, may vary a lot among different compounds. The intralayer B-X bonds are much more covalent and stronger than the mostly ionic interlayer A-X bonds in AZn2Sb2 and ACd2Sb2, whereas the intralayer bonds in AMg2X2 are only slightly stronger than the largely ionic interlayer bonds (see Table 1 and Fig. 2a, b, f). This is further confirmed by the similar reduced density gradient distributions of the three nonequivalent bonds from the non-covalent interaction analysis101 in Mg3Sb2 (Fig. 2g). The chemical bonding analysis may be conducive to understanding transport properties. The mostly ionic feature with partial covalency (high polarity) of chemical bonds explains the intrinsically low carrier mobility36,102 in p-type Mg3Sb2 and CaMg2Sb2. However, the high carrier mobility in AMg2Bi2 (A = Mg, Ca, Eu, and Yb)26,41 cannot be understood from chemical bonding, though it could be attributed to the bipolar effect or the change in carrier scattering time induced by defects.
The electron density ratio \(\rho _{{\mathrm{intra}}}{\mathrm{/}}\rho _{{\mathrm{inter}}}\) is devised as a simplified parameter measuring the degree of anisotropy of the chemical bonding network in the Mg3Sb2-related structures,79 where \(\rho _{{\mathrm{intra}}}\) and \(\rho _{{\mathrm{inter}}}\) represent the electron density values at BCPs of the intralayer and interlayer bonds, respectively. Unlike AZn2Sb2 and ACd2Sb2 with clear anisotropic chemical bonding networks (\(\rho _{{\mathrm{intra}}}{\mathrm{/}}\rho _{{\mathrm{inter}}} > 2\)), the AMg2X2 compounds show nearly isotropic 3D chemical bonding networks with \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\) typically being smaller than 1.5 and often close to unity (Table 1). The nearly isotropic 3D bonding networks can be used to understand the nearly isotropic structural and thermal properties especially lattice thermal conductivities in AMg2X2,79 which will be discussed later.
Crystal field orbital splitting, orbital degeneracy, and p-type electronic transport
In principle, the intrinsic electronic transport of a material is determined almost exclusively by the degeneracy and curvature of the electronic bands at the band edges. The Seebeck coefficient is typically determined by the density of states effective mass md* = Nv2/3ms*,1,103 where Nv represents the valley degeneracy of the electronic bands and ms* denotes the effective mass of a single valley. As proposed by Goldsmid,1,103 the optimum electrical transport performance can be expressed as being proportional to the weighted mobility μ(md*/me)3/2, where μ and me represent the carrier mobility and the mass of an electron, respectively. Considering the case with predominant acoustic phonon scattering or alloy scattering, the weighted mobility can be simplified and written as Nv/mc* (Nv/ms* for an isotropic band), where mc* is the conductivity effective mass. It is clear that excellent electrical performance requires a high valley degeneracy as well as a light conductivity effective mass. Increasing valley degeneracy (including orbital degeneracy) has long been recognized as an efficient way to improve electrical transport performance through enhancing the Seebeck coefficient without explicitly decreasing the carrier mobility when the intervalley scattering is insignificant.4,6,9,103,104 Below we will introduce how electrical transport can be rationalized through enhancing orbital degeneracy via minimizing the orbital splitting energy105 in p-type AB2X2 compounds with the CaAl2Si2-type structure.
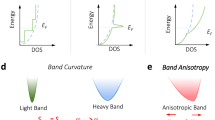
In CaAl2Si2-type compounds, the valence band maximum (VBM) located at the Г point shows p orbital characteristics of the anions (Fig. 3a, b). Unlike the triply degenerate p orbitals at the Γ point in cubic structures protected by the point symmetry, the pz orbital, due to the crystal field effect, is usually well separated from the px,y orbitals in the trigonal CaAl2Si2-type structure. Accordingly, the three-fold degenerate valence band at the Γ point splits into a nondegenerate band Γ(pz) and a doubly degenerate band Γ(px,y), where Γ(px,y) and Γ(pz) are typically a heavy and light hole band, respectively (Fig. 3a–d).9,105 The crystal field splitting energy Δ between them is defined as Δ = E(Γ(px,y)) − E(Γ(pz)), where the orbital degeneracy is effectively increased when the splitting energy approaches zero. The basic idea of the orbital engineering approach proposed by Zhang et al.105 is to minimize the splitting energy Δ with the aim to maximize orbital degeneracy and thereby optimize electrical transport performance. This concept is well confirmed by the Boltzmann transport calculations as well as the experimental observations, which show peak values in power factor as Δ ≈ 0 (see Fig. 3e, f). As the thermal conductivities of most promising CaAl2Si2-type TE compounds are comparable especially at high temperatures, the strongly enhanced power factor results in peak zT values at Δ ≈ 0.105 The strong correlation between experimental power factors and Δ also indicates the minimal intervalley scattering in different AB2X2 compounds, which ensures the success of this approach. Spin–orbit coupling (SOC) is found to lift the degeneracy of the px,y band, but will not change the main idea of the approach. Accordingly, a simple selection rule, i.e., maintaining the valence band splitting energy around zero (zero-Δ rule), is proposed to optimize the electrical transport performance.
a Schematic diagram of orbital engineering to realize three-fold degenerate p orbitals in CaAl2Si2-type compounds. Nondegenerate band Γ(pz) and doubly degenerate band Γ(px,y) are mainly composed of pz and px,y orbitals from the anions, respectively. Δ denotes the crystal field splitting energy between px,y and pz orbitals at the Γ point. b Band structure of Mg3Sb2 by TB-mBJ potential without spin–orbit coupling. The p orbitals of Sb are projected on the band structure. c, d Partial charge densities of the valence bands c Γ(pz) (isovalue: 0.06 e Å−3) and d Γ(px,y) (isovalue: 0.13 e Å−3) at the Γ point in Mg3Sb2. e The theoretical power factor at 300 K versus Δ of Mg3Sb2 at various hole concentrations p. The red curve shows the best values corresponding to the optimum carrier concentrations. Data points for unoptimized carrier concentrations fill up the pink area right below the red curve. f The experimental power factors14,15,17,18,19,20,21,22,23,24,25,26,27,39,40,41 of reported CaAl2Si2-type compounds at 600 K as a function of Δ. Red points represent the alloys18,21 with zT larger than unity at high temperatures. a, b, e, f are adapted with permission from ref. 105, CC-BY-4.0
The zero-Δ selection rule may be combined with the band gap criterion Eg < 1.5 eV with the aim to search for promising TE candidates from a rich variety of CaAl2Si2(AB2X2)-type compounds. The band gaps of these AB2X2 materials show a clear decreasing trend when the electronegativity difference between B and X decreases, which may be rationalized by a decrease of the energy between the atomic orbitals of B and X and an increase in band widths using the molecular orbital scheme.106,107 However, it should be noted that SOC also plays an important role in decreasing energy gaps of Bi-based compounds.105 Excellent TE properties are well confirmed in Sb-based and Bi-based compounds such as EuZn2Sb2,17 EuMg2Bi2,29 and YbZn2Sb215 with small Δ values close to zero and ideal band gaps. In particular, Shuai et al. reported strongly enhanced power factors in EuMg2Bi2 with a nearly zero Δ value compared with those of CaMg2Bi2 and YbMg2Bi2 with Δ values largely deviating from zero,29 which provides a solid confirmation of the zero-Δ rule. Though simple and powerful, the zero-Δ rule only has resulted in the discovery of a few potential TE candidates.
Materials design and optimization requires effective approaches for manipulating crystal field orbital splitting energy. The hybridizations or overlap integrals of p orbitals control their splitting energy at the VBM. In general, tuning structural parameters (for instance, the interlayer distance, bond lengths and angles, and c/a) by the crystal deformation is able to effectively manipulate the orbital interactions and thereby the orbital splitting energy. In principle, crystal deformation can be induced by both external and internal forces. External forces include physical pressure and strain effect, while internal forces involve chemical doping or forming solid solutions.105 Two efficient approaches, solid solution map and biaxial strain engineering, can be used to realize the manipulation of Δ.105 Using the solid solution map with calculated Δ values versus lattice parameter or band gap Eg, one can conveniently choose two or more compounds with positive and negative Δ values to form a solid solution with the desirable Δ value of zero, which leads to excellent electronic transport performance (see Fig. 4a). Since alloying is also conducive to reducing the thermal conductivity owing to the point defect scattering, strongly enhanced zT values can generally be achieved using this approach. This powerful strategy is confirmed in several alloys including YbCd1.6Zn0.4Sb2,18 EuZn1.8Cd0.2Sb2,21 Eu0.2Yb0.2Ca0.6Mg2Bi2,29 YbCd1.5Zn0.5Sb2,35 and Ca0.99Na0.01MgZnSb236 with superior TE power factors and zTs. In particular, Wood et al. revealed the valence band crossing in CaMg2Sb2-CaZn2Sb2 alloys, which combines with reducing thermal conductivities by the alloy scattering to result in a peak zT of 0.87 at 850 K.36 As shown by Wang et al., optimizing electrical transport performance induced by minimizing the p orbital splitting energy as well as minimizing the lattice thermal conductivity by the point defect scattering leads to an optimal zT of ~1.3 at 700 K in the YbCd2−xZnxSb2 alloys.35 In addition to the approach of forming solid solutions, biaxial strain engineering can be applied to continuously tune Δ so as to optimize electrical transport performance in thin-film TE materials (see Fig. 4b). A general principle for optimizing TE performance via biaxial strain is that for compounds with negative (positive) Δ value the compressive (tensile) biaxial strain is more effective.
Solid solution map and biaxial strain engineering for materials design. a Calculated Δ versus the lattice constant a in CaAl2Si2-type compounds with Eg < 1.5 eV. Reported thermal conductivities14,15,17,18,19,20,21,22,23,24,25,26,27,39,40,41 at 500 K are shown in color bar. The stars correspond to solid solutions YbCd1.5Zn0.5Sb2 and EuZn1.75Cd0.25Sb2 with nearly zero Δ values. b Δ as a function of biaxial strain ε in two representative CaAl2Si2-type compounds Mg3Sb2 and CaZn2Sb2. Biaxial strain ε is defined as (a − a0)/a0 × 100%, where a0 and a are the in-plane lattice parameters with unstrained and strained states, respectively. Figure is adapted from ref. 105, CC-BY-4.0
Since most semiconductors show p-orbital characteristics at the valence band edges, the orbital engineering approach can easily be extended to other structures with the same orbital splitting features. One notable extension is the earlier reported pseudocubic approach in chalcopyrite structures,9 where the crystal field splitting energy can be directly linked to the structural deformation parameter η = c/2a. Moreover, the approach can be extended to layered metal dichalcogenides and lithium intercalated metal dichalcogenides.105 Therefore, the orbital engineering approach with physical and chemical insights based on the underlying atomic orbitals enriches band engineering and may substantially accelerate the screening and design of new TE materials from layered or noncubic compounds.
Multi-valley conduction bands, complex Fermi surface, and exceptional n-type electronic transport
The valley degeneracy is defined as the number of different carrier pockets (for the same type of carriers) existing at a given energy level. In general, the valley degeneracy of an individual electronic band can be defined as Nv = Nv,symNv,band, where Nv,sym is the number of symmetry equivalent positions in the Brillouin zone for a given k point at which the electronic band occurs and Nv,band represents the number of electronic bands degenerate at the same k point and energy level. For the high-symmetry Γ point located at the center of the Brillouin zone, Nv,sym = 1. The orbital engineering approach105 discussed in the previous section is such a case. Although the band degeneracy Nv,band of 3 at the Γ point (at the VBM) can be achieved via tuning the splitting energy of p orbitals, Nv,sym of 1 at the Γ point limits the overall valley degeneracy to be Nv ≤ 3. It is thereby clear that a high valley degeneracy requires not only a high band degeneracy Nv,band but also a high Nv,sym. In order to obtain a high Nv,sym value, we need a high-symmetry Brillouin zone as well as a band extremum that occurs at a low-symmetry k point.1,2 Below we will introduce the multi-valley band behavior as the electronic origin of the extraordinary electrical transport performance in n-type Mg3Sb2-based TE materials, which exhibit a unique near-edge conduction band minimum (CBM) at a low-symmetry k point with a high Nv,sym of 6.
The electronic structure of Mg3Sb2 has been studied using different theoretical methods in various reports. Traditional functionals such as GGA108 or LDA109 are known to underestimate band gaps while recent calculations54,74 with the TB-mBJ potential110 gives more accurate band gaps close to the experimental values. However, band structures in all earlier reports are typically calculated along the high-symmetry k paths,74,75,76,77 which often overlook the actual CBM at the low-symmetry k point. As revealed in the recent calculations including SOC by Zhang et al.,57 the accurate CBM in Mg3Sb2 is located at the CB1 point (0, 0.417, 0.333), which is along the M*–L* line inside the first Brillouin zone (see Fig. 5a). Mg3Sb2 shows an indirect band gap of ~0.6 eV with the VBM at the Γ point and the CBM at the CB1 point. Moreover, there is a secondary band minimum located just above the CB1 extremum at the K point. The small energy difference \(\Delta E_{{\mathrm{K - CB}}_{\mathrm{1}}}\) of 0.078 eV between the two band minima CB1 and K suggests that they might even be treated as effectively converged at elevated temperatures because of the thermal broadening of Fermi function. Inspired by the biaxial strain engineering105 in p-type compounds, Li et al. revealed that n-type electrical performance of Mg3Sb2 can also be optimized by tuning \(\Delta E_{{\mathrm{K - CB}}_{\mathrm{1}}}\) towards zero via the biaxial strain.111
a Band structure and partial density of states of Mg3Sb2 by TB-mBJ potential with SOC. b High-symmetry k points and k paths in the Brillouin zone. c Multiple carrier pockets shown in Fermi surface of n-type Mg3Sb2 for an energy level at 0.1 eV above the conduction band minimum CB1. d One highly anisotropic carrier pocket at the Г point shown in Fermi surface of p-type Mg3Sb2 corresponding to an energy level at 0.1 eV below the valence band maximum. a, b are adapted with permission from ref. 57, American Chemical Society. c, d are adapted with permission from ref. 54, CC-BY-4.0
The iso-energy Fermi surface provides an intuitive shortcut to understand the multi-valley conduction bands in Mg3Sb2 (see Fig. 5b–d). In contrast to only one highly anisotropic hole pocket of the VBM at the Γ point, the Fermi surface of n-type Mg3Sb2, corresponding to an energy level 0.1 eV above the conduction band minimum CB1, shows 6 isolated full electron pockets inside the Brillouin zone and 6 one-third pockets at the K point (see Fig. 5c).54 As a result, the valley degeneracies for the conduction band minima CB1 and K are, respectively, 6 and 2, which may be added up to 8 when they are effectively converged. Such a high valley degeneracy is comparable to many state-of-the-art TE materials such as (Bi,Sb)2Te3 and PbTe.2,103
The complex Fermi surface is conducive to good TE performance. As proposed by Gibbs et al.,112 the complexity of Fermi surface may be characterized by the simplified parameter Nv*K*, where Nv* and K* represent the effective valley degeneracy and anisotropy parameter of a single carrier pocket, respectively. In general, the higher the Nv*K*, the better the electrical transport performance. In addition to the high valley degeneracy, the complexity of Fermi surface in n-type Mg3Sb2 is clearly reflected in the ellipsoidal-like carrier pockets at the CB1, indicating a clear anisotropic feature. For every individual carrier pocket at the CB1 point, the effective mass is anisotropic along one longitudinal (elongated) and two transverse directions in k space with m||* = 0.55me, m⊥,1* = 0.21me, and m⊥,2* = 0.28me, although when averaging over the six equivalent carrier pockets the overall average effective mass tensor follows the crystal symmetry and shows a nearly isotropic feature with mkx* = mky* = (0.21 × 4 + 0.55 × 2)/6 = 0.32me and mkz* = 0.28me.113 Regarding the Fermi surface complexity, it is crucial to take into account the contribution of the anisotropic feature of the single carrier pocket at the CB1, which shows a moderate anisotropy parameter K = m||*/(m⊥,1*m⊥,2*)1/2 ≈ 2.3.
The Fermi surface complexity factor Nv*K* can be calculated by (md*/mc*)3/2,112,114 where mc* is evaluated using the carrier density n and theoretical electrical conductivity σ/τ from a BoltzTraP115 calculation by mc* = ne2/(σ/τ) under the constant carrier’s scattering time approximation.116 md* is estimated by fitting the Seebeck coefficient from Boltzmann transport calculations applied to the full DFT band structure using the single band model. The theoretical calculations reveal a peak Nv*K* of ~19 in n-type Mg3Sb2, much higher than that of p-type Mg3Sb2 (see Fig. 6a).113 This is consistent with both the theoretical and experimental results (see Fig. 6b, c), displaying significantly enhanced power factors for n-type doping in comparison with those for p-type doping in Mg3Sb2-based materials. The high Fermi surface complexity, as well as the superior n-type electrical transport performance, can be attributed to a combined effect of the large md* induced by the high valley degeneracy, the light conductivity effective mass, and the carrier pocket anisotropy (see Fig. 6a). The n-type transport of Mg3Sb2 shows a primary contribution from the nontrivial band minimum CB1 as well as a certain contribution from the secondary band minimum at the K point. This multiple band behavior in n-type Mg3Sb2 is confirmed by the result from the BoltzTraP calculation that Mg3SbBi with a much larger \(\Delta E_{{\mathrm{K - CB}}_{\mathrm{1}}}\) of ~0.18 eV shows lower Seebeck coefficients in comparison with those of Mg3Sb2 (see Fig. 6d).
a Conductivity effective mass mc*, density of states (DOS) effective mass md*, and Fermi surface complexity factor Nv*K* estimated from BoltzTraP115 as a function of Hall carrier concentration (nH) in p-type and n-type Mg3Sb2.113 nH is estimated by 1/eRH, where RH is Hall coefficient. b Theoretical power factor54 at 300 K from BoltzTraP versus nH in Mg3Sb2. c Experimental power factor versus Hall mobility at room temperature for reported p-type and n-type Mg3Sb2-based materials. d Seebeck coefficient values (|α|) as a function of nH at 300 K. The black and red solid lines correspond to the prediction of p-type and n-type Mg3Sb2 from BoltzTraP, taken from ref. 54. The orange dash-dotted line shows the prediction by a single parabolic band (SPB) model with a DOS effective mass equal to that of the CB1 band at the CBM of Mg3Sb2.54 The red dashed line represents the theoretical prediction of n-type Mg3SbBi taken from ref. 113. In c, d, experimental data of p-doped Mg3Sb2 and n-doped Mg3+xSb1.5Bi0.5 are taken from refs. 41,45,46,47,49,50 and refs. 54,55,56,57,58,59,60,63,64,66,67,71, respectively
Though the multiple conduction band behavior was also found in ternary AMg2X2 and AZn2Sb2,117 the unique conduction band CB1 with a six-fold valley degeneracy only exists in binary Mg3X2 (X = As, Sb, and Bi). As shown by the theoretical calculations of Sun et al., the unique CBM at the CB1 point in Mg3Sb2 might possibly be explained by the bonding states between Mg1 and Mg2 atoms.118 However, the very large interatomic distance between Mg1 and Mg2 (~3.7 Å) indicates that the orbital interaction between Mg1 and Mg2, if any, should be very weak. Moreover, the calculations were only conducted for Mg3Sb2 without a systematic comparison with many other ternary compounds. The underlying origin of the unique six-fold CB1 band minimum in Mg3X2 still requires further investigation. In Mg3X2, the band gaps decrease from Mg3As2 (1.6 eV) to Mg3Sb2 (0.6 eV) to Mg3Bi2 (semimetal). It is found that doping or substituting on the anion sites will not destroy the favorable conduction band minimum CB1 since the conduction bands are mainly contributed by the electronic states of Mg (Fig. 5a).54,57 Therefore, n-doped Mg3Sb2, Mg3As2, as well as the solid solutions Mg3Sb2−xBix, Mg3Sb2−xAsx, and Mg3As2−xBix with suitable band gaps are predicted to show promising electrical transport performance if they are properly doped on the anion sites.54
Experimentally, we are especially interested in n-type Mg3Sb2−xBix with the energy gaps Eg ≤ ~0.6 eV within ~10kBT (T = 300–725 K), which is generally the suitable value for a good TE material.119 DFT calculations113 with the TB-mBJ potential reveal the accurate conduction band alignments of Mg3Sb2−xBix alloys (see Fig. 7a), which makes a correction to the simple schematic plot by Imasato et al.62 The Mg3Bi2 alloying results in a moderate increase in the energy separation between conduction band minima K and CB1, making the contribution of the secondary band minimum K to electronic transport insignificant. The conduction band minimum Г (Nv = 1) shows a noticeable downward shift with the Mg3Bi2 alloying. However, the band minimum Г is too far from the band minimum CB1 to play a significant role in n-type electronic transport before the band-gap closure (x ≈ 1.7). As the composition of Mg3Bi2 increases in Mg3Sb2−xBix solid solutions, the band gap is linearly reduced from ~0.6 eV (x = 0) to ~0.24 eV (x = 1) to zero (semimetal, x ≈ 1.7), indicating an increasing detrimental bipolar contribution. In addition, the narrowing of the band gap, accompanied by the increasing near-edge band widths, results in the decreasing band effective masses. This is confirmed in several reports62,111 as well as the theoretical result113 by BoltzTraP (Fig. 7b), which shows a clear decreasing trend in mc* of near-edge conduction bands from Mg3Sb2 to Mg3SbBi to Mg3Bi2.
a The conduction band alignments in Mg3Sb2−xBix (x = 0, 1, and 2) solid solutions by the TB-mBJ method with SOC.113 It should be noted that the three conduction band minima are located at different k points (i.e., CB1, K, and Г). The band alignment is calculated by aligning the Mg-1s core levels of different compounds. The valence band maximum of Mg3Sb2 at the Г point is set to 0 eV. b Conductivity effective mass versus Hall carrier concentration simulated from BoltzTraP for n-type Mg3Sb2−xBix.113 SOC and noSOC denote the cases with and without SOC, respectively. It is clear that SOC has a negligible effect on near-edge conduction bands in Mg3Sb2−xBix because the CBM is dominated by electronic states of the light element Mg (see Fig. 5a). Figure is adapted from ref. 113
Although the Mg3Bi2 alloying lowers the Seebeck coefficient due to the lighter conduction band mass and the weaker contribution from the secondary band minimum K, it is conducive to increasing the carrier mobility and weighted mobility as the conductivity effective mass mc* is decreased (see Fig. 7). An appropriate amount of the Mg3Bi2 alloying (x ≤ 1) would be ideal for n-doped Mg3Sb2−xBix to have an enhanced weighted mobility for superior TE performance without a noticeable bipolar effect at elevated temperatures. This is consistent with the widely reported compositions of n-doped Mg3Sb2−xBix (x = 0, 0.5, and 1) with excellent high-temperature TE performance. An increasing bipolar effect with increasing Bi content in Mg3Sb2−xBix is expected to shift the peak zT to lower temperatures, but an improved zT at low temperatures may be achieved owing to the lighter conductivity effective mass. Thus more efforts should be made to investigate n-type Mg3Sb2−xBix with a wider range of compositions (x = 0–1.7). It will also be interesting to design a functionally graded n-doped Mg3Sb2−xBix (x = 0–1.7) system for TE applications. Below we give a brief overview of the recent experimental reports on n-doped Mg3Sb2−xBix.
As shown originally by Pedersen53 in 2012 (see the link in ref. 53 for details) and later reported by Tamaki et al.55 and Zhang et al.54, an exceptionally high n-type TE performance can be achieved in Mg3Sb1.5Bi0.5 through Te doping on the anion site with or without excess Mg. Zhang et al.54 reported n-type Mg3Sb1.5–0.5xBi0.5–0.5xTex with varying Te content and no excess Mg synthesized by combining arc melting and spark plasma sintering (SPS) at 1123 K based on the reproduction and improvement of the original work by Pedersen.53 The samples with an optimal zT of 0.56–1.65 at 300–725 K reported by Zhang et al.54 show the intrinsic temperature-dependent mobility with the dominant acoustic phonon scattering at low temperatures. Tamaki et al.55 reported n-type Mg3.2Sb1.5Bi0.49Te0.01 with a large nominal excess Mg prepared using ball milling followed by SPS pressing at 873 K, which exhibits low carrier mobility and TE performance below 500 K due to impurity scattering. Later, two approaches were applied to improve the mobility and power factor at low temperatures (Fig. 6c) by tuning the carrier scattering mechanism from the ionized impurity scattering to the acoustic-phonon-dominated mixed scattering. One approach is to dope Mg3.2Sb1.5Bi0.5 with the transition metals (i.e., Nb, Fe, Co, Hf, Ta, and Mn) on the Mg sites and the Te dopant on the anion sites using a pressing temperature of 927 K as reported by Shuai et al.,56 Mao et al.,58 and Chen et al.66 Following the earlier work of Zhang et al.,54 the other approach is to increase the pressing temperature to 1123 or 1073 K, which was also confirmed in the studies by Kanno et al.64 and Mao et al.59 Combining the above two approaches, Chen et al.71 was able to further improve the room-temperature zT and average zT in n-type porosity-mediated Mg3.225Mn0.025Sb1.5Bi0.49Te0.01 with an SPS pressing temperature of 1073 K. Regarding the underlying mechanism, Mao et al.59 suggested that the increasing mobility might be due to reduction of the number of Mg vacancies, whereas Kuo et al.120 proposed an illuminating two-phase model that explains the experimental observation by the larger grain sizes reducing the grain boundary electrical resistance under a higher pressing temperature. In addition, enhanced low-temperature TE performance was shown in n-type Mg3.2SbBi0.99Te0.01 and Te-doped Mg3Sb0.6Bi1.4 by Imasato et al.62,70 and Mg3.02SbyBi1.99−yTe0.01 (y = 1.1–1.5) by Shu et al.68 with increasing Bi content, which can be attributed to the lighter conductivity effective mass. Regarding n-type dopants other than the Te element, Se57 and S60 on the anion site as well as La67 and Y69 on the cation site have also been experimentally explored in Mg3+xSb1.5Bi0.5. With continuous experimental efforts, a low-temperature zT of ~0.9 at 323 K and a peak zT of ~1.85 at 723 K have been achieved so far in n-type (Mn, Te)-codoped Mg3Sb1.5Bi0.5.66,71 In addition to the intensive developments in TE performance, the thermal stability of n-type Te-doped Mg3Sb1.5Bi0.5 was investigated by Jørgensen et al.72 using synchrotron powder X-ray diffraction and X-ray total scattering, where a clear evolution of bismuth as a secondary phase was observed in the powdered sample during thermal cycling.
Several experimental and theoretical efforts54,60,61,63,65,121,122 reveal that a large amount of excess Mg is not needed for achieving n-type properties as claimed by Tamaki et al.55. A tiny amount of excess Mg or even no excess Mg is required to realize n-type properties as long as great care is taken to avoid the Mg loss during the synthesis, consistent with the earlier report without excess Mg by Zhang et al.54 The samples with smaller amounts of excess or no excess Mg even show better performance and stability than those with large amounts of excess Mg.63,122 Various reports with different amounts of excess Mg may arise from the difficulty in preparing these materials without the Mg loss due to the high reactivity, easy oxidation, and high vapor pressure of Mg.
Defect-controlled carrier transport, electronegativity, and bonding character
Nearly all CaAl2Si2-type compounds, except CaAl2Si2123 and SrAl2Si2124, have been reported as intrinsically p-type, which is attributed to the intrinsic point defects pinning the chemical potential close to the valence bands. According to the intrinsic defect calculations,80,125 the cation vacancy at the A site is the most energetically stable point defect in all AZn2Sb2 (A = Ca, Sr, Eu, and Yb) compounds, which leads to the persistent p-type behavior in these compounds. Pure Mg3Sb2 is intrinsically p-type, and many attempts at doping or substituting with a series of elements result in p-type behaviors as well.38,39,40,41,42,43,44,45,46,47,48,49,50,52,102,126,127,128,129,130 This is because of the low formation energies of the negatively charged Mg vacancies (\(V_{{\mathrm{Mg1}}}^{{\mathrm{2 - }}}\) and \(V_{{\mathrm{Mg2}}}^{{\mathrm{2 - }}}\)) (Fig. 8), which prevent the chemical potential from moving close to the conduction bands for the Sb-rich condition.55,61 The n-type doping in Mg3Sb2-based materials is challenging and only recently discovered to be successful.51,53,54,55 Several defect calculations55,61,121 were conducted to explain the n-type behavior in Mg3Sb2 under the Mg-excess condition. The results vary from different methods applied in defect energy calculations. Ohno et al.61 concluded from calculations using the PBE functional108 with finite-size and band-gap corrections that under the Mg-excess condition the Mg vacancy suppression rather than the Mg interstitial55 is responsible for n-type properties (Fig. 8), while Chong et al.121 based on more accurate calculations with the HSE06 functional131 revealed that the role of the excess Mg for achieving n-type behavior is to compensate the electronic charge of the defect complex (VMg2 + Mgi)1−. It is clear that defect calculations are usually sensitive to the applied theoretical methods; however, the robust relative trend of defect formation energies is supposed to render insightful guidance for the carrier transport. Below we introduce guidelines for the defect-controlled carrier transport with respect to the electronegativity in p-type AZn2Sb2- and n-type Mg3Sb2-based materials.
a, b Defect formation energies of intrinsic point defects as well as the extrinsic defect TeSb under a Sb-excess (\(\Delta \mu _{{\mathrm{Sb}}}{\mathrm{ = 0}}\)) and b Mg-excess (\(\Delta \mu _{{\mathrm{Mg}}}{\mathrm{ = 0}}\)) conditions. Figure is adapted with permission from ref. 61, Elsevier
In p-type AZn2Sb2 (A = Ca, Sr, Eu, and Yb), the experimental carrier density increases with increasing electronegativity132 of A (Fig. 9a). As revealed by Pomrehn et al.80 using DFT calculations, the increasing carrier density can be rationalized by the decreasing trend in the vacancy formation energy at the cation A site with increasing electronegativity (Fig. 9b). The relationship between the vacancy defect-formation energy and electronegativity might be related to the relative energy cost of the electron transfer influenced by the bond strength between the cation A and the anion, which in turn affects the vacancy formation energies. In general, the formation of the vacancy is easier when the bonding is weak. However, in order to explain the deduction, accurate calculations should be conducted to characterize the bond strength between A and Sb. Though the underlying mechanism regarding the bond strength is complicated, the results provide insight on tuning carrier density by alloying on the A site with elements of varying electronegativities. This idea is well confirmed in many significant reports15,19,23,32 on optimizing p-type AB2X2 TEs, where excellent zTs were achieved owing to the carrier density optimization and thermal conductivity reduction via alloying at the A site.
Defect-controlled carrier density. a The experimental Hall carrier concentration15,16,17,20,95 at room temperature versus the electronegativity of the cation A (A = Sr, Eu, Ca, and Yb) in p-type AZn2Sb2. The Allred–Rochow scale132 of the electronegativity is used. b Defect formation energy of the cation vacancy in AZn2Sb2. The respective conduction band minima of different compounds are shown in the colored dashed lines. c Hall carrier concentration54,57,60 at 300 K as a function of the electronegativity difference \(\left| {\chi _{\mathrm{Q}}-\chi _{{\mathrm{Mg}}}} \right|\) (Q = S, Se, and Te) in n-type chalcogen-doped Mg3Sb1.5Bi0.5. The Pauling scale133 of the electronegativity is adopted. d Defect formation energies of extrinsic defects QSb as well as the intrinsic defects VMg1 and VMg2 under Mg-rich and Sb-rich conditions. a, b and c, d are adapted with permissions, respectively, from refs. 80 and 60, Wiley-VCH
Defect-controlled carrier transport with the insight from the electronegativity133 difference was explored in n-type Mg3Sb1.5Bi0.5 with the chalcogens Q (S, Se, and Te) as electron dopants.60 Experimentally, it is found that both the maximum attainable carrier concentration and mobility increase with decreasing electronegativity difference \(\left| {{\mathrm{\chi }}_{\mathrm{Q}} - {\mathrm{\chi }}_{{\mathrm{Mg}}}} \right|\) between the chalcogen dopants Q and Mg (Fig. 9c). Using DFT calculations, Zhang et al.60 revealed that the improving carrier concentration is caused by the increasing doping limit induced by the lower extrinsic defect-formation energy (Fig. 9d), which may be explained by the stronger bonding between the dopant and the matrix with decreasing \(\left| {{\mathrm{\chi }}_{\mathrm{Q}} - {\mathrm{\chi }}_{{\mathrm{Mg}}}} \right|\). Moreover, the enhanced mobility is attributed to the smaller effective mass of conduction bands originating from the enhanced bond covalency with decreasing electronegativity difference,134,135 which is supported by the decreasing theoretical density of states. According to the above trends, using dopants with a small electronegativity difference compared to its bonding partner was proposed as a guiding rule for efficient n-type doping in Mg3Sb2-based compounds.60 As confirmed in extrinsic defect calculations for n-type Mg3Sb2 by Gorai et al.,136 this guideline was found to work pretty well for the anion site doping but might not work well for the cation site doping due to the strong charge compensation and complex competing phases involved. Moreover, theoretical calculations by Gorai et al.136,137 suggested the group-3 elements including La, Y, and Sc as effective n-type cation dopants for Mg3Sb2, which was confirmed by the recent experiments67,69 of La-doped and Y-doped Mg3+xSb1.5Bi0.5.
Anharmonic phonon modes and low lattice thermal conductivity
Reported lattice thermal conductivities14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,71 κL at room temperature in CaAl2Si2-type TE compounds span a relatively wide range from ~0.6 W m−1 K−1 in CaCd2Sb2 to ~4.5 W m−1 K−1 in CaMg2Sb2. Among them, Mg3Sb2 with a low density of ~4.0 g cm−3 shows an intrinsically low room-temperature κL of about 1.0–1.5 W m−1 K−1,40,45,46 comparable to those of the state-of-the-art TE materials PbTe and Bi2Te3.1,138,139 A small difference (~15%) in κL is found between single crystalline and polycrystalline Mg3Sb2 samples,40,102,130 suggesting that the grain boundary scattering83 does not play a significant role in phonon transport. Though alloying is often applied to further reducing lattice thermal conductivity through point defect scattering, the intrinsically low thermal conductivity induced by the phonon–phonon scattering is indispensable to the exceptionally high zT in n-type Mg3Sb2-based materials. Below we will discuss the origin of the intrinsically low lattice thermal conductivity in Mg3Sb2 from first principles.
The lattice thermal conductivity of Mg3Sb2 and the two ternary analogs (CaMg2Sb2 and CaMg2Bi2) was calculated by Zhang et al.84 using first-principles Boltzmann transport calculations considering the anharmonic phonon–phonon scattering with the ShengBTE140 code. Importantly, the theoretical result shows a noticeable decrease in κL from CaMg2Sb2 and CaMg2Bi2 to Mg3Sb2, which is in good agreement with experiments (see Fig. 10a). It is found that the phonon lifetime plays a key role in reducing κL in Mg3Sb2.82,84 In general, the phonon lifetime for intrinsic crystalline materials is dominated by the three-phonon anharmonic scattering process. As revealed in Fig. 10b, the calculated anharmonic scattering rates of Mg3Sb2 are considerably larger than those of CaMg2Sb2 and CaMg2Bi2 in the low- and mid-frequency regions (<5.5 THz). In particular, the anharmonic scattering rates of Mg3Sb2 display one peak at 0.7–1.04 THz and two peaks in the mid-frequency region of 3.3–4.8 THz, which are about (or even more than) one order of magnitude larger than those of ternary compounds within the same frequency regions. The peak in anharmonic scattering rates of Mg3Sb2 at 0.7–1.04 THz is typically induced by a unique feature in phonon dispersion in comparison with ternary compounds as pointed out earlier by Peng et al.,82 i.e., much softer transverse acoustic phonon modes at the M (~0.71 THz), L (~0.81 THz), and A (~1.04 THz) points characterized by very large mode Grüneisen parameters (Fig. 10c, d). In particular, the soft transverse acoustic phonon mode at the L point exhibits the largest negative mode Grüneisen parameter. A similar behavior was also found in Mg3Bi2, where the phonon calculations often show negative (imaginary) frequencies for transverse acoustic branches at the L point. In addition, the peak anharmonic scattering rates of Mg3Sb2 at 3.3–4.8 THz in Mg3Sb2 are induced by the anharmonic optical phonon modes within this frequency region with much larger mode Grüneisen parameters than those of CaMg2Sb2 and CaMg2Bi2 (Fig. 10d). Therefore, the soft transverse acoustic phonon modes within 0.7–1.04 THz as well as the anharmonic optical modes within 3.3–4.8 THz contribute to the large peak values in the anharmonic scattering rates and thereby intrinsically low lattice thermal conductivity in Mg3Sb2.
a The experimental and theoretical lattice thermal conductivities. The theoretical and experimental data are taken from refs. 79,84 and 26,36,40,43, respectively. Nano-Mg3Sb2 denotes the nanostructured Mg3Sb2 sample43 prepared by the high-energy ball milling method. b The calculated anharmonic scattering rates of Mg3Sb2, CaMg2Sb2, and CaMg2Bi2.84 c Phonon band structure and density of states of Mg3Sb2. Adapted with permission from ref. 79, CC-BY-4.0. The mode Grüneisen parameters are projected on the phonon band structure.84 Curve width indicates the relative magnitude of the mode Grüneisen parameter. The positive and negative mode Grüneisen parameters are colored in red and blue, respectively. d Mode Grüneisen parameters of Mg3Sb2, CaMg2Sb2, and CaMg2Bi2.84 For the theoretical results shown here, the longitudinal optical/transverse optical (LO/TO) splitting is not considered since the LO/TO splitting only has a minor effect on the results.84 The readers are referred to ref. 82 for the results considering the LO/TO splitting
The unique soft transverse phonon modes are related to the structural instability in Mg3Sb2 and Mg3Bi2, which is well confirmed in their less negative formation enthalpies141,142 compared with other CaAl2Si2-type compounds (Fig. 11a). As claimed by Peng et al.,82 the soft transverse acoustic phonon modes in Mg3Sb2 and Mg3Bi2 may be attributed to the unstable Mg1 in the octahedral sites as judged by the small cation-to-anion ionic radius ratio below the stability limit (0.414), which results from the small ionic radius of Mg (Fig. 11b). Although the insight proposed by Peng et al.82 is illuminating, it cannot explain why Mg3As2, which also exhibits a cation-to-anion ionic radius ratio below the stability limit, does not show soft transverse acoustic phonon modes. Clearly, the ionic radius ratio rule is somewhat oversimplified by assuming purely ionic bonding with a complete charge transfer. In reality, chemical bonds in Mg3X2 show certain degrees of covalency with incomplete charge transfers (see Table 1), which will result in a larger effective ionic radius of Mg1 and probably push the cation-to-anion ionic radius ratio above the stability limit.
a Formation enthalpy versus the electronegativity difference ΔEN in AB2X2 (CaAl2Si2-type) compounds. ΔEN is calculated by the difference between the electronegativity of the anion X and the average electronegativity of the atoms A and B. Formation enthalpy values are taken from MaterialsProject.org.141,142 b The cation-to-anion ionic radius ratio rcation/ranion versus the ionic radius of the cation A at the octahedral site in AMg2X2. The minimum stability limit of rcation/ranion for the octahedral site is 0.414. The data points for Mg3Sb2 and Mg3Bi2 are colored in pink. Adapted with permissions from ref. 82, Elsevier. c Experimental isotropic atomic displacement parameters of Mg3Sb2. d The potential energy curves for the nonequivalent atoms in Mg3Sb2 displacing along the a and c directions. c, d are reproduced with permissions from ref. 79, CC-BY-4.0
The instability of Mg1 is well confirmed in the relatively larger atomic displacement parameter and flatter potential energy landscape of Mg1 compared to those of Mg2 in Mg3Sb2 (Fig. 11c, d),79 which might be attributed to the relatively weaker Mg1-Sb bond as well as the larger void space of the octahedral site of Mg1. However, the unstable Mg1 at the octahedral site better explains the anharmonic optical modes at 3.3–4.8 THz rather than the soft transverse acoustic modes since the anharmonic optical modes are mainly contributed by the motion of Mg1 while the acoustic phonon modes are dominated by the vibration of Sb (Fig. 10b, c). It is more likely that the unstable or weak chemical bonds lead to the soft transverse acoustic phonon modes in Mg3Sb2 and Mg3Bi2.
The soft transverse acoustic phonon modes in Mg3Sb2 and Mg3Bi2 involve the interlayer shearing motion, which results in their reported soft shear moduli.82,97 However, whether the soft shear modes are mainly induced by the weak interlayer Mg1-Sb (or Mg1-Bi) bond requires further confirmation. Quantitative chemical bonding analysis based on the electron density98 is reliable in comparing different chemical bonds in the same compound, but it cannot be used for reliably comparing the relative strength of chemical bonds in different compounds. Therefore, other computational methods should be applied to quantitatively characterize and compare the relative strength of the interlayer A-X bonds in different compounds. Furthermore, other factors such as the difference in atomic masses should also be considered. Overall, more comprehensive work should be conducted in the future to reveal the origin of these unique soft transverse modes in Mg3Sb2 and Mg3Bi2.
Electronic and thermal transport anisotropy
Experimentally, the anisotropy of the transport properties in bulk TE materials can vary a lot between different reports48,102,130,143 since it is sensitive to the factors such as the sample quality, crystal alignment, and preferred orientations. Therefore, here we will focus on the anisotropy of electrical and thermal transport based on the perfect crystal structure from the theoretical point of view. In general, only electronic states near the Fermi level contribute to the electronic transport. Under the constant and isotropic carrier scattering time assumption, the anisotropy of electrical conductivity is completely determined by the anisotropy in carrier mobility and thereby the anisotropy in the inverse effective mass tensor, which can be intuitively understood from the anisotropy of the atomic orbitals interactions. For p-type transport in Mg3Sb2, the electronic states at the VBM are mainly comprised of the pz orbitals of the Sb atoms.105 The anisotropic pz orbitals show much stronger orbital overlaps along the c axis, which results in much larger band dispersion, smaller effective mass, and larger electrical conductivity in comparison to those along the a axis. This is confirmed by the calculated strong anisotropy in the electrical conductivity for p-type Mg3Sb2 with σxx/σzz being much smaller than unity (see Fig. 12a), consistent with the experimental reports.102 In contrast, for n-type transport in Mg3Sb2, the electronic states at the CBM are dominated by spherical s orbitals of the Mg atoms,55,57 which generally result in nearly isotropic features in orbital interactions, average effective mass tensor, and thereby electrical conductivity (Fig. 12a).144 For the Seebeck coefficient, both p-type and n-type Mg3Sb2 show a nearly isotropic feature even though the effective mass tensor is anisotropic (Fig. 12a). This may be attributed to the notion, which was proposed by Parker et al.,114 that the anisotropic effective mass results in isotropic Seebeck coefficient as long as the electronic band is assumed parabolic, the carrier scattering time is assumed only dependent on energy, and the bipolar effect is insignificant. Moreover, as investigated by Sun et al.,117 the Seebeck coefficients of several ternary compounds AMg2X2 and AZn2Sb2 (A = Ca, Sr, Ba; X = Sb, Bi) also show isotropic features when there is no bipolar effect, whereas the n-type electrical conductivities in these compounds show anisotropic features due to the anisotropic average effective mass tensors induced by the anisotropic electron pockets, which may be understood from the anisotropic orbital interactions induced by the increased contributions of anisotropic p and d orbitals to the CBM.
a Anisotropy of the calculated electronic transport properties including Seebeck coefficient (αxx/αzz) and electrical conductivity (σxx/σzz) as a function of Hall carrier concentration in p-type and n-type Mg3Sb2. The data are calculated in this work using the previous computational methods54. b Anisotropy of the theoretical lattice thermal conductivity κa/κc as a function of the anisotropy of the chemical bonding network characterized by \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\). b is reproduced with permissions from ref. 79, CC-BY-4.0
In general, the thermal transport anisotropy is related to the anisotropy of the chemical bonding network.79,145 In Mg3Sb2 and related structures, the anisotropy of the chemical bonding network can be quantified by the intralayer-to-interlayer bond-strength ratio \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\) based on electron density.79 A nearly linear correlation between the anisotropy ratio κa/κc of lattice thermal conductivity and \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\) indicates that \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\) can be adopted as an indicator measuring the anisotropy of lattice thermal conductivity in Mg3Sb2-related materials (see Fig. 12b). For AMg2X2 compounds, the nearly isotropic 3D bonding network with \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\) ≈ 1 results in the nearly isotropic features in phonon dispersion, group velocity, Grüneisen parameter, and ultimately lattice thermal conductivity.79 However, the lattice thermal conductivity in AZn2Sb2 is relatively anisotropic due to the anisotropic chemical bonding network with \(\rho _{{\mathrm{intra}}}/\rho _{{\mathrm{inter}}}\,{>}\, 2\).
Summary and outlook
In this review, we have explored many illuminating insights such as orbital overlap, orbital degeneracy, orbital splitting energy, valley degeneracy, effective mass, carrier pocket anisotropy, Fermi surface complexity, point defects, electronegativity, and bond covalency for understanding electronic and thermal transport of Mg3Sb2 and related CaAl2Si2-type TEs. Although current insights for the materials design have been very helpful to date, many challenges need to be addressed in order to achieve further development. Below we present some challenges and possible future directions in both theoretical and experimental aspects.
Theoretically, critical challenges include the difficulties for current computations to describe the carrier scattering time, doping, temperature effects, defects, and disorder in experiments. One typical example of these challenges can be seen in electrical transport calculations under the rigid band and constant carrier’s scattering time approximation, which assumes electronic bands and scattering time being insensitive to temperature and doping. In reality, doping on the cation sites and varying carrier scattering mechanisms result in the deviation from theory and the scatter of experimental data in the Pisarenko plot of the Seebeck coefficient versus carrier density (see Fig. 6d). Another example can be found in the situation where the high carrier mobility cannot be understood from the band structure. In several compounds containing rare earth elements, the carrier’s scattering time is responsible for the intrinsically high carrier mobility, whereas the detailed mechanism requires further investigation. In n-type Mg3Sb2−xBix, changing carrier scattering behavior can improve carrier mobility by doping with transition metals on the Mg sites or increasing the pressing temperature, but the underlying mechanism is still under debate.
In addition, the insights into the origin of valence and conduction band alignments, how thermal expansion affects band alignments, and the origin of carrier pocket anisotropy remain unclear. The underlying mechanism on why the favorable six-fold CBM at the low-symmetry CB1 point only exists in binary Mg3X2 requires further investigation. For defect calculations, more potential n-type dopants need to be predicted for the experimental validations. Regarding thermal transport, further efforts are required to elucidate the origin of the soft transverse acoustic phonon modes in Mg3Sb2 and Mg3Bi2 that remains obscurely understood to date. Whether the soft transverse acoustic modes are induced by the weak interlayer interaction should be carefully examined. The critical challenge here for computations is how to quantitatively and reliably compare the strength of interlayer interactions in different compounds. In addition to this possible notion, the impact of other factors such as the atomic mass difference on phonon transport should be examined.
Experimentally, the reported compositions are mainly limited to Sb- and Bi-based compounds, while As- and P-based compounds remain largely unexplored. According to the solid solution compound map, there are still many unexplored alloying combinations showing the potential to achieve superior p-type electrical properties with minimal crystal orbital splitting energy values. Moreover, excellent n-type properties have only been discovered in Mg3Sb2−xBix with few effective electron dopants such as the chalcogens, Y, and La. Hence, other effective n-type dopants for Mg3Sb2−xBix as well as other potential material systems such as Mg3Sb2−xAsx and Mg3Bi2−xAsx for n-type doping need to be explored in the future. Despite the outstanding TE performance shown in n-type Mg3Sb2-based materials, the long-term thermal stability under a temperature gradient and an electrical current should be addressed as an essential step toward the practical applications.
References
Goldsmid, H. J. Thermoelectric Refrigeration (Plenum, New York, 1964).
Nolas, G. S, Sharp, J. & Goldsmid, H. J. Thermoelectrics: Basic Principles and New Materials Developments (Springer, New York, 2001).
Snyder, G. J., Christensen, M., Nishibori, E., Caillat, T. & Iversen, B. B. Disordered zinc in Zn4Sb3 with phonon-glass and electron-crystal thermoelectric properties. Nat. Mater. 3, 458–463 (2004).
Zaitsev, V. K. et al. Highly effective Mg2Si1-xSnx thermoelectrics. Phys. Rev. B 74, 045207 (2006).
Christensen, M. et al. Avoided crossing of rattler modes in thermoelectric materials. Nat. Mater. 7, 811–815 (2008).
Pei, Y. et al. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 473, 66–69 (2011).
Biswas, K. et al. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 489, 414–418 (2012).
Liu, H. et al. Copper ion liquid-like thermoelectrics. Nat. Mater. 11, 422–425 (2012).
Zhang, J. et al. High-performance pseudocubic thermoelectric materials from non-cubic chalcopyrite compounds. Adv. Mater. 26, 3848–3853 (2014).
Zhao, L.-D. et al. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 508, 373–377 (2014).
Zheng, C. & Hoffmann, R. Complementary local and extended views of bonding in the ThCr2Si2 and CaAl2Si2 structures. J. Solid State Chem. 72, 58–71 (1988).
Zheng, C., Hoffmann, R., Nesper, R. & Von Schnering, H. G. Site preferences and bond length differences in CaAl2Si2-type Zintl compounds. J. Am. Chem. Soc. 108, 1876–1884 (1986).
Verbrugge, D. M. & Zytveld, J. B. J. V. Electronic properties of liquid MgSb. J. Non-Cryst. Solids 156–158, 736 (1993).
Stark, D. & Snyder, G. J. The synthesis of CaZn2Sb2 and its thermoelectric properties. In Proc. 21th Inter. Conf. Thermoelectrics 181–184 (Institute of Electrical and Electronics Engineers Inc., Piscataway, NJ, 2002).
Gascoin, F., Ottensmann, S., Stark, D., Haïle, S. M. & Snyder, G. J. Zintl phases as thermoelectric materials: tuned transport properties of the compounds CaxYb1-xZn2Sb2. Adv. Funct. Mater. 15, 1860–1864 (2005).
Yu, C. et al. Improved thermoelectric performance in the Zintl phase compounds YbZn2-xMnxSb2 via isoelectronic substitution in the anionic framework. J. Appl. Phys. 104, 013705 (2008).
Zhang, H. et al. A new type of thermoelectric material, EuZn2Sb2. J. Chem. Phys. 129, 164713 (2008).
Wang, X.-J. et al. Synthesis and high thermoelectric efficiency of Zintl phase YbCd2−xZnxSb2. Appl. Phys. Lett. 94, 092106 (2009).
Cao, Q.-G. et al. Zintl phase Yb1-xCaxCd2Sb2 with tunable thermoelectric properties induced by cation substitution. J. Appl. Phys. 107, 053714 (2010).
Toberer, E. S., May, A. F., Melot, B. C., Flage-Larsen, E. & Snyder, G. J. Electronic structure and transport in thermoelectric compounds AZn2Sb2 (A=Sr, Ca, Yb, Eu). Dalton Trans. 39, 1046–1054 (2010).
Zhang, H. et al. Thermoelectric properties of Eu(Zn1-xCdx)2Sb2. Dalton Trans. 39, 1101–1104 (2010).
Zhang, H. et al. Synthesis and properties of CaCd2Sb2 and EuCd2Sb2. Intermetallics 18, 193–198 (2010).
Zhang, H. et al. Thermoelectric properties of YbxEu1−xCd2Sb2. J. Chem. Phys. 133, 194701 (2010).
Zhang, H. et al. Thermoelectric properties of polycrystalline SrZn2Sb2 prepared by spark plasma sintering. J. Electron. Mater. 39, 1772–1776 (2010).
Guo, K. et al. Enhanced thermoelectric figure of merit of Zintl phase YbCd2-xMnxSb2 by chemical substitution. Eur. J. Inorg. Chem. 2011, 4043–4048 (2011).
May, A. F. et al. Thermoelectric transport properties of CaMg2Bi2, EuMg2Bi2, and YbMg2Bi2. Phys. Rev. B 85, 035202 (2012).
Zevalkink, A. et al. Nonstoichiometry in the Zintl phase Yb1-δZn2Sb2 as a route to thermoelectric optimization. Chem. Mater. 26, 5710–5717 (2014).
Min, W., Guo, K., Wang, J. & Zhao, J. Effect of manganese doping on the thermoelectric properties of Zintl phase EuCd2Sb2. J. Rare Earth. 33, 1093–1097 (2015).
Shuai, J. et al. Higher thermoelectric performance of Zintl phases (Eu0.5Yb0.5)1-xCaxMg2Bi2 by band engineering and strain fluctuation. Proc. Natl Acad. Sci. USA 113, E4125–E4132 (2016).
Shuai, J. et al. Thermoelectric properties of Zintl compound Ca1-xNaxMg2Bi1.98. Appl. Phys. Lett. 108, 183901 (2016).
Shuai, J. et al. Thermoelectric properties of Bi-based Zintl compounds Ca1-xYbxMg2Bi2. J. Mater. Chem. A 4, 4312–4320 (2016).
Wubieneh, T. A., Wei, P.-C., Yeh, C.-C., Chen, S.-y & Chen, Y.-Y. Thermoelectric properties of Zintl phase compounds of Ca1-xEuxZn2Sb2 (0≤x≤1). J. Electron. Mater. 45, 1942–1946 (2016).
Takagiwa, Y. et al. Thermoelectric properties of EuZn2Sb2 Zintl compounds: zT enhancement through Yb substitution for Eu. J. Alloy. Compd. 703, 73–79 (2017).
Wang, X. et al. Single parabolic band transport in p-type EuZn2Sb2 thermoelectrics. J. Mater. Chem. A 5, 24185–24192 (2017).
Wang, X. et al. Orbital alignment for high performance thermoelectric YbCd2Sb2 alloys. Chem. Mater. 30, 5339–5345 (2018).
Wood, M., Aydemir, U., Ohno, S. & Snyder, G. J. Observation of valence band crossing: the thermoelectric properties of CaZn2Sb2–CaMg2Sb2 solid solution. J. Mater. Chem. A 6, 9437–9444 (2018).
Kunioka, H. et al. Thermoelectric properties of (Ba,K)Cd2As2 crystallized in the CaAl2Si2-type structure. Dalton Trans. 47, 16205–16210 (2018).
Kajikawa, T., Kimura, N. & Yokoyama, T. Thermoelectric properties of intermetallic compounds: Mg3Bi2 and Mg3Sb2 for medium temperature range thermoelectric elements. In Proc. 22th Inter. Conf. Thermoelectrics 305–308 (Institute of Electrical and Electronics Engineers Inc., Piscataway, NJ, 2003).
Condron, C. L., Kauzlarich, S. M., Gascoin, F. & Snyder, G. J. Thermoelectric properties and microstructure of Mg3Sb2. J. Solid State Chem. 179, 2252–2257 (2006).
Bhardwaj, A. et al. Mg3Sb2-based Zintl compound: a non-toxic, inexpensive and abundant thermoelectric material for power generation. RSC Adv. 3, 8504–8516 (2013).
Ponnambalam, V. & Morelli, D. On the thermoelectric properties of Zintl compounds Mg3Bi2-xPnx (Pn=P and Sb). J. Electron. Mater. 42, 1307–1312 (2013).
Bhardwaj, A. & Misra, D. K. Enhancing thermoelectric properties of a p-type Mg3Sb2-based Zintl phase compound by Pb substitution in the anionic framework. RSC Adv. 4, 34552–34560 (2014).
Bhardwaj, A., Chauhan, N. S. & Misra, D. K. Significantly enhanced thermoelectric figure of merit of p-type Mg3Sb2-based Zintl phase compounds via nanostructuring and employing high energy mechanical milling coupled with spark plasma sintering. J. Mater. Chem. A 3, 10777–10786 (2015).
Bhardwaj, A., Shukla, A. K., Dhakate, S. R. & Misra, D. K. Graphene boosts thermoelectric performance of a Zintl phase compound. RSC Adv. 5, 11058–11070 (2015).
Shuai, J. et al. Thermoelectric properties of Na-doped Zintl compound: Mg3−xNaxSb2. Acta Mater. 93, 187–193 (2015).
Song, L., Zhang, J. & Iversen, B. B. Simultaneous improvement of power factor and thermal conductivity via Ag doping in p-type Mg3Sb2 thermoelectric materials. J. Mater. Chem. A 5, 4932–4939 (2017).
Chen, C. et al. Enhanced thermoelectric performance of p-type Mg3Sb2 by lithium doping and its tunability in an anionic framework. J. Mater. Sci. 53, 16001–16009 (2018).
Lv, F. et al. Isotropic Mg3Sb2 compound prepared by solid-state reaction and ball milling combined with spark plasma sintering. J. Mater. Sci. 53, 8039–8048 (2018).
Ren, Z. S. et al. Significantly enhanced thermoelectric properties of p-type Mg3Sb2 via co-doping of Na and Zn. Acta Mater. 143, 265–271 (2018).
Wang, H. et al. Enhanced thermoelectric performance in p-type Mg3Sb2 via lithium doping. Chin. Phys. B 27, 047212 (2018).
Kim, S. et al. Thermoelectric properties of Mn-doped Mg-Sb single crystals. J. Mater. Chem. A 2, 12311–12316 (2014).
Bhardwaj, A. et al. Tuning the carrier concentration using Zintl chemistry in Mg3Sb2, and its implications for thermoelectric figure-of-merit. Phys. Chem. Chem. Phys. 18, 6191–6200 (2016).
Pedersen, S. H. Thermoelectric Properties of Zintl Compounds Mg 3 Sb 2-x Bi x. (Chemistry Project for Master’s Degree, Department of Chemistry, Aarhus University, 2012). The thesis is available at http://chem.au.dk/fileadmin/cmc.chem.au.dk/pictures_new_homepage/Pedersen_S_H_-_Thermoelectric_Properties_of_Zintl_Compounds_Mg3Sb2-xBix.pdf.
Zhang, J. et al. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat. Commun. 8, 13901 (2017).
Tamaki, H., Sato, H. K. & Kanno, T. Isotropic conduction network and defect chemistry in Mg3+δSb2-based layered Zintl compounds with high thermoelectric performance. Adv. Mater. 28, 10182–10187 (2016).
Shuai, J. et al. Tuning the carrier scattering mechanism to effectively improve the thermoelectric properties. Energy Environ. Sci. 10, 799–807 (2017).
Zhang, J., Song, L., Mamakhel, A., Jørgensen, M. R. V. & Iversen, B. B. High-performance low-cost n-type Se-doped Mg3Sb2-based Zintl compounds for thermoelectric application. Chem. Mater. 29, 5371–5383 (2017).
Mao, J. et al. Manipulation of ionized impurity scattering for achieving high thermoelectric performance in n-type Mg3Sb2-based materials. Proc. Natl Acad. Sci. USA 114, 10548–10553 (2017).
Mao, J. et al. Defect engineering for realizing high thermoelectric performance in n-type Mg3Sb2-based materials. ACS Energy Lett. 2, 2245–2250 (2017).
Zhang, J., Song, L., Borup, K. A., Jørgensen, M. R. V. & Iversen, B. B. New insight on tuning electrical transport properties via chalcogen doping in n-type Mg3Sb2-based thermoelectric materials. Adv. Energy Mater. 8, 1702776 (2018).
Ohno, S. et al. Phase boundary mapping to obtain n-type Mg3Sb2-based thermoelectrics. Joule 2, 141–154 (2018).
Imasato, K., Kang, S. D., Ohno, S. & Snyder, G. J. Band engineering in Mg3Sb2 by alloying with Mg3Bi2 for enhanced thermoelectric performance. Mater. Horiz. 5, 59–64 (2018).
Imasato, K., Ohno, S., Kang, S. D. & Snyder, G. J. Improving the thermoelectric performance in Mg3+xSb1.5Bi0.49Te0.01 by reducing excess Mg. APL Mater. 6, 016106 (2018).
Kanno, T. et al. Enhancement of average thermoelectric figure of merit by increasing the grain-size of Mg3.2Sb1.5Bi0.49Te0.01. Appl. Phys. Lett. 112, 033903 (2018).
Shuai, J. et al. Significant role of Mg stoichiometry in designing high thermoelectric performance for Mg3(Sb,Bi)2-based n-type Zintls. J. Am. Chem. Soc. 140, 1910–1915 (2018).
Chen, X. et al. Extraordinary thermoelectric performance in n-type manganese doped Mg3Sb2 Zintl: High band degeneracy, tuned carrier scattering mechanism and hierarchical microstructure. Nano Energy 52, 246–255 (2018).
Imasato, K., Wood, M., Kuo, J. J. & Snyder, G. J. Improved stability and high thermoelectric performance through cation site doping in n-type La-doped Mg3Sb1.5Bi0.5. J. Mater. Chem. A 6, 19941–19946 (2018).
Shu, R. et al. Mg3+δSbxBi2−x family: a promising substitute for the state-of-the-art n-type thermoelectric materials near room temperature. Adv. Funct. Mater. 29, 1807235 (2019).
Song, S. W. et al. Joint effect of magnesium and yttrium on enhancing thermoelectric properties of n-type Zintl Mg3+δY0.02Sb1.5Bi0.5. Mater. Today Phys. 8, 25–33 (2019).
Imasato, K., Kang, S. D. & Snyder, G. J. Exceptional thermoelectric performance in Mg3Sb0.6Bi1.4 for low-grade waste heat recovery. Energy Environ. Sci. 12, 965–971 (2019).
Chen, X. et al. Porosity-mediated high-performance thermoelectric Materials. Preprint at https://arxiv.org/abs/1810.03356 (2018).
Jørgensen, L. R., Zhang, J. W., Zeuthen, C. B. & Iversen, B. B. Thermal stability of Mg3Sb1.475Bi0.475Te0.05 high performance n-type thermoelectric investigated through powder X-ray diffraction and pair distribution function analysis. J. Mater. Chem. A 6, 17171–17176 (2018).
Song, L., Zhang, J. & Iversen, B. B. Thermal stability of p-type Ag-doped Mg3Sb2 thermoelectric materials investigated by powder X-ray diffraction. Phys. Chem. Chem. Phys. 21, 4295–4305 (2019).
Singh, D. J. & Parker, D. Electronic and transport properties of zintl phase AeMg2Pn2, Ae=Ca,Sr,Ba, Pn=As,Sb,Bi in relation to Mg3Sb2. J. Appl. Phys. 114, 143703 (2013).
Watson, L. M., Marshall, C. A. W. & Cardoso, C. P. On the electronic structure of the semiconducting compounds Mg3Bi2 and Mg3Sb2. J. Phys. F: Met. Phys. 14, 113 (1984).
Xu, R., Groot, R. Ad & Lugt, Wvd Resistivities and band structures of alkaline-earth-pnictide systems. J. Phys. Condens. Matter 5, 7551 (1993).
Imai, Y. & Watanabe, A. Electronic structures of Mg3Pn2 (Pn=N, P, As, Sb and Bi) and Ca3N2 calculated by a first-principle pseudopotential method. J. Mater. Sci. 41, 2435–2441 (2006).
Burdett, J. K. & Miller, G. J. Fragment formalism in main-group solids: applications to aluminum boride (AlB2), calcium aluminum silicide (CaAl2Si2), barium-aluminum (BaAl4), and related materials. Chem. Mater 2, 12–26 (1990).
Zhang, J., Song, L., Sist, M., Tolborg, K. & Iversen, B. B. Chemical bonding origin of the unexpected isotropic physical properties in thermoelectric Mg3Sb2 and related materials. Nat. Commun. 9, 4716 (2018).
Pomrehn, G. S., Zevalkink, A., Zeier, W. G., van de Walle, A. & Snyder, G. J. Defect-controlled electronic properties in AZn2Sb2 Zintl phases. Angew. Chem. Int. Ed. 53, 3422–3426 (2014).
Tani, J.-i, Takahashi, M. & Kido, H. Lattice dynamics and elastic properties of Mg3As2 and Mg3Sb2 compounds from first-principles calculations. Phys. B 405, 4219–4225 (2010).
Peng, W., Petretto, G., Rignanese, G. M., Hautier, G. & Zevalkink, A. An unlikely route to low lattice thermal conductivity: small atoms in a simple layered structure. Joule 2, 1879–1893 (2018).
Maccioni, M. B., Farris, R. & Fiorentini, V. Ab initio thermal conductivity of thermoelectric Mg3Sb2: evidence for dominant extrinsic effects. Phys. Rev. B 98, 220301(R) (2018).
Zhang, J. & Iversen, B. B. Lattice dynamics and lattice thermal conductivity of Mg3Sb2, in preparation (2019).
Gladyshevskii, E. I., Krypyakevych, P. I. & Bodak, O. I. The crystal structure of the compound CaAl2Si2 and its analogues. Ukr. Fiz. Zh. 12, 447–452 (1967).
Martinez-Ripoll, M., Haase, A. & Brauer, G. The crystal structure of α-Mg3Sb2. Acta Cryst. B 30, 2006–2009 (1974).
Zintl, E. & Husemann, E. Bindungsart und gitterbau binärer magnesiumverbindungen. Z. Phys. Chem. 21B, 138–155 (1933).
Grube, G. & Bornhak, R. Das Zustandsdiagramm magnesium-antimon. Z. Elektrochem. 40, 140–142 (1934).
Grube, G., Mohr, L. & Bornhak, R. Elektrische leitfähigkeit und zustandsdiagramm bei binären legierungen. 10. mitteilung. Das system magnesium-wismut. Z. Elektrochem. 40, 143–150 (1934).
Barnes, A. C., Guo, C. & Howells, W. S. Fast-ion conduction and the structure of β-Mg3Bi2. J. Phys. Condens. Matter 6, L467–L471 (1994).
Zintl, E. Intermetallische verbindungen. Angew. Chem. 52, 1–6 (1939).
Schäfer, H., Eisenmann, B. & Müller, W. Zintl phases: transitions between metallic and ionic bonding. Angew. Chem. Int. Ed. 12, 694–712 (1973).
Kauzlarich, S. M., Brown, S. R. & Jeffrey Snyder, G. Zintl phases for thermoelectric devices. Dalton Trans. 0, 2099–2107 (2007).
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nat. Mater. 7, 105–114 (2008).
May, A. F. et al. Properties of single crystalline AZn2Sb2 (A=Ca,Eu,Yb). J. Appl. Phys. 111, 033708 (2012).
Alemany, P., Llunell, M. & Canadell, E. Roles of cations, electronegativity difference, and anionic interlayer interactions in the metallic versus nonmetallic character of Zintl phases related to arsenic. J. Comput. Chem. 29, 2144–2153 (2008).
Li, G. et al. Deformation mechanisms in high-efficiency thermoelectric layered Zintl compounds. J. Mater. Chem. A 5, 9050–9059 (2017).
Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford University Press, Oxford, 1990).
Mori-Sánchez, P., Pendás, A. M. & Luaña, V. A classification of covalent, ionic, and metallic solids based on the electron density. J. Am. Chem. Soc. 124, 14721–14723 (2002).
Gatti, C. Chemical bonding in crystals: new directions. Z. Krist. 220, 399–457 (2005).
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010).
Soo Hyun, K. et al. Thermoelectric properties of Mg3Sb2-xBix single crystals grown by Bridgman method. Mater. Res. Express 2, 055903 (2015).
Goldsmid, H. J. Introduction to Thermoelectricity (Springer, Berlin, 2009).
Liu, W. et al. Convergence of conduction bands as a means of enhancing thermoelectric performance of n-type Mg2Si1-xSnx solid solutions. Phys. Rev. Lett. 108, 166601 (2012).
Zhang, J. et al. Designing high-performance layered thermoelectric materials through orbital engineering. Nat. Commun. 7, 10892 (2016).
Cox, P. A. The Electronic Structure and Chemistry of Solids (Oxford University Press, Oxford, 1987).
Peng, W., Chanakian, S. & Zevalkink, A. Crystal chemistry and thermoelectric transport of layered AM2X2 compounds. Inorg. Chem. Front. 5, 1744–1759 (2018).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P. & Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 23, 5048–5079 (1981).
Tran, F. & Blaha, P. Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 102, 226401 (2009).
Li, J. et al. Designing high-performance n-type Mg3Sb2-based thermoelectric materials through forming solid solutions and biaxial strain. J. Mater. Chem. A 6, 20454–20462 (2018).
Gibbs, Z. M. et al. Effective mass and Fermi surface complexity factor from ab initio band structure calculations. npj Comput. Mater 3, 8 (2017).
Zhang, J. & Iversen, B. B. Fermi surface complexity, effective mass, and conduction band alignment in n-type thermoelectric Mg3Sb2-xBix from first principles calculations. Preprint at https://arxiv.org/abs/1907.02263 (2019).
Parker, D. S., May, A. F. & Singh, D. J. Benefits of carrier-pocket anisotropy to thermoelectric performance: the case of p-type AgBiSe2. Phys. Rev. Appl. 3, 064003 (2015).
Madsen, G. K. H. & Singh, D. J. BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 175, 67–71 (2006).
Lykke, L., Iversen, B. B. & Madsen, G. K. H. Electronic structure and transport in the low-temperature thermoelectric CsBi4Te6: Semiclassical transport equations. Phys. Rev. B 73, 195121 (2006).
Sun, J. & Singh, D. J. Thermoelectric properties of AMg2X2, AZn2Sb2 (A=Ca, Sr, Ba; X=Sb, Bi), and Ba2ZnX2 (X=Sb, Bi) Zintl compounds. J. Mater. Chem. A 5, 8499–8509 (2017).
Sun, X. et al. Achieving band convergence by tuning the bonding ionicity in n-type Mg3Sb2. J. Comput. Chem. 40, 1693–1700 (2019).
Mahan, G. D. & Sofo, J. O. The best thermoelectric. Proc. Natl Acad. Sci. USA 93, 7436–7439 (1996).
Kuo, J. J. et al. Grain boundary dominated charge transport in Mg3Sb2-based compounds. Energy Environ. Sci. 11, 429–434 (2018).
Chong, X. et al. Understanding the intrinsic p-type behavior and phase stability of thermoelectric α-Mg3Sb2. ACS Appl. Energy Mater. 1, 6600–6608 (2018).
Lee, K., Bux, S. K. & Fleurial, J.-P. Thermal stability and transport properties of n-type Mg 3 Sb 2-x Bi x. Poster presented at 36th Inter. Conf. Thermoelectrics, Pasadena, CA, USA (2017).
Imai, M., Abe, H. & Yamada, K. Electrical properties of single-crystalline CaAl2Si2. Inorg. Chem. 43, 5186–5188 (2004).
Kauzlarich, S. M., Condron, C. L., Wassei, J. K., Ikeda, T. & Snyder, G. J. Structure and high-temperature thermoelectric properties of SrAl2Si2. J. Solid State Chem. 182, 240–245 (2009).
Bjerg, L., Madsen, G. K. H. & Iversen, B. B. Ab initio calculations of intrinsic point defects in ZnSb. Chem. Mater. 24, 2111–2116 (2012).
Xin, H. X. & Qin, X. Y. Electrical and thermoelectric properties of nanocrystal substitutional semiconductor alloys Mg3(BixSb1-x)2 prepared by mechanical alloying. J. Phys. D Appl. Phys. 39, 5331 (2006).
Xin, H. X., Qin, X. Y., Zhu, X. G. & Liu, Y. Temperature dependence of electrical resistivity for nanocrystalline Mg3+xSb2 prepared by mechanical alloying. J. Phys. D Appl. Phys. 39, 375 (2006).
Ahmadpour, F., Kolodiazhnyi, T. & Mozharivskyj, Y. Structural and physical properties of Mg3-xZnxSb2 (x=0–1.34). J. Solid State Chem. 180, 2420–2428 (2007).
Zhang, K. X., Qin, X. Y., Xin, H. X., Li, H. J. & Zhang, J. Transport and thermoelectric properties of nanocrystal substitutional semiconductor alloys (Mg1-xCdx)3Sb2 doped with Ag. J. Alloy. Compd. 484, 498–504 (2009).
Xin, J. et al. Growth and transport properties of Mg3X2 (X=Sb, Bi) single crystals. Mater. Today Phys. 7, 61–68 (2018).
Paier, J. et al. Screened hybrid density functionals applied to solids. J. Chem. Phys. 124, 154709 (2006).
Allred, A. L. & Rochow, E. G. A scale of electronegativity based on electrostatic force. J. Inorg. Nucl. Chem. 5, 264–268 (1958).
Pauling, L. The nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 54, 3570–3582 (1932).
Slack, G. A. in CRC Handbook of Thermoelectrics (ed. Rowe, D. M.) 407 (CRC, Boca Raton, FL, 1995).
Zeier, W. G. et al. Thinking like a chemist: intuition in thermoelectric materials. Angew. Chem. Int. Ed. 55, 6826–6841 (2016).
Gorai, P., Ortiz, B. R., Toberer, E. S. & Stevanovic, V. Investigation of n-type doping strategies for Mg3Sb2. J. Mater. Chem. A 6, 13806–13815 (2018).
Gorai, P., Toberer, E. S. & Stevanović, V. Effective n-type doping of Mg3Sb2 with group-3 elements. J. Appl. Phys. 125, 025105 (2019).
Ravich, Y. I., Efimova, B. A. & Smirnov, I. A. Semiconducting Lead Chalcogenides (Plenum Press, New York, 1970).
Rowe, D. M. (ed.) CRC Handbook of Thermoelectrics (CRC, Boca Raton, FL, 1995).
Li, W., Carrete, J., A. Katcho, N. & Mingo, N. ShengBTE: a solver of the Boltzmann transport equation for phonons. Comput. Phys. Commun. 185, 1747–1758 (2014).
Jain, A. et al. The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Jain, A. et al. Formation enthalpies by mixing GGA and GGA+U calculations. Phys. Rev. B 84, 045115 (2011).
Song, S. W. et al. Study on anisotropy of n-type Mg3Sb2-based thermoelectric materials. Appl. Phys. Lett. 112, 092103 (2018).
Li, J. et al. Computational prediction of a high ZT of n-type Mg3Sb2-based compounds with isotropic thermoelectric conduction performance. Phys. Chem. Chem. Phys. 20, 7686–7693 (2018).
Grin, Y. Inhomogeneity and anisotropy of chemical bonding and thermoelectric properties of materials. J. Solid State Chem. 274, 329–336 (2019).
Acknowledgements
We thank K.F.F. Fischer for discussions and comments. This work was supported by the Danish National Research Foundation (Center for Materials Crystallography, DNRF93) and the Danish Center for Scientific Computing. The numerical results presented in this work were obtained at the Center for Scientific Computing, Aarhus. Affiliation with the Center for Integrated Materials Research (iMAT) at Aarhus University is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
J.Z. wrote the manuscript with the inputs and suggestions from B.B.I. L.S. collected the reported experimental data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Song, L. & Iversen, B.B. Insights into the design of thermoelectric Mg3Sb2 and its analogs by combining theory and experiment. npj Comput Mater 5, 76 (2019). https://doi.org/10.1038/s41524-019-0215-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-019-0215-y
This article is cited by
-
Efficient first-principles electronic transport approach to complex band structure materials: the case of n-type Mg3Sb2
npj Computational Materials (2024)
-
Maximizing the performance of n-type Mg3Bi2 based materials for room-temperature power generation and thermoelectric cooling
Nature Communications (2022)
-
Demonstration of valley anisotropy utilized to enhance the thermoelectric power factor
Nature Communications (2021)
-
Restructured single parabolic band model for quick analysis in thermoelectricity
npj Computational Materials (2021)
-
Optoelectronic, thermodynamic and vibrational properties of intermetallic MgAl2Ge2: a first-principles study
SN Applied Sciences (2021)