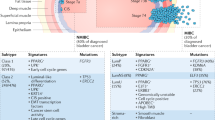
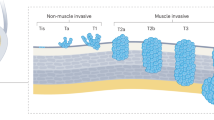
Abstract
Bladder cancer is a heterogeneous group of tumours with at least 40 histological subgroups. Patients with localized disease can be cured with surgical resection or radiotherapy, but such curative options are limited in the setting of recurrent disease or distant spread, in which case systemic therapy is used to control disease and palliate symptoms. Cytotoxic chemotherapy has been the mainstay of treatment for advanced bladder cancer, but high-quality evidence is lacking to inform the management of rare subgroups that are often excluded from studies. Advances in molecular pathology, the development of targeted therapies and the resurgence of immunotherapy have led to the reclassification of bladder cancer subgroups and rigorous efforts to define predictive biomarkers for cancer therapies. In this Review, we present the current evidence for the management of conventional, variant and divergent urothelial cancer subtypes, as well as non-urothelial bladder cancers, and discuss how the integration of genomic, transcriptomic and proteomic characterization of bladder cancer could guide future therapies.
Key points
-
Combining sequencing and transcriptomic technologies might improve the identification of clinically relevant bladder cancer subgroups.
-
Molecular subtyping has helped us to identify bladder tumours that respond well to cytotoxic chemotherapy.
-
Targeted therapies have had a limited role in bladder cancer management, but the next generation of specific targets are showing promise, as exemplified by fibroblast growth factor receptor 3.
-
Immune checkpoint inhibition leads to deep and durable responses in a small subgroup of patients.
-
Composite molecular signatures are showing promise as predictors of treatment response and should be tested in prospective clinical trials.
-
Real-time serial biopsies during the course of treatment will be required to help direct therapy in an evolving tumour landscape.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ferlay, J. et al. (eds) GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0: IARC CancerBase No. 11 (International Agency for Research on Cancer, Lyon, 2012).
Alanee, S. et al. Update of the International Consultation on Urological Diseases on bladder cancer 2018: non-urothelial cancers of the urinary bladder. World J. Urol. 37, 107–114 (2019).
Humphrey, P. A., Moch, H., Cubilla, A. L., Ulbright, T. M. & Reuter, V. E. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur. Urol. 70, 106–119 (2016).
Garraway, L. A. Genomics-driven oncology: framework for an emerging paradigm. J. Clin. Oncol. 31, 1806–1814 (2013).
Dinney, C. P. et al. Focus on bladder cancer. Cancer Cell 6, 111–116 (2004).
Dadhania, V. et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine 12, 105–117 (2016).
Choi, W. et al. Genetic alterations in the molecular subtypes of bladder cancer: illustration in the Cancer Genome Atlas Dataset. Eur. Urol. 72, 354–365 (2017).
Damrauer, J. S. et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl Acad. Sci. USA 111, 3110–3115 (2014).
Sjodahl, G. et al. Toward a molecular pathologic classification of urothelial carcinoma. Am. J. Pathol. 183, 681–691 (2013).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014).
Biton, A. et al. Independent component analysis uncovers the landscape of the bladder tumor transcriptome and reveals insights into luminal and basal subtypes. Cell Rep. 9, 1235–1245 (2014).
Lerner, S. P. et al. Bladder cancer molecular taxonomy: summary from a consensus meeting. Bladder Cancer 2, 37–47 (2016).
Robertson, A. G. et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171, 540–556 (2017).
Chan, K. et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat. Genet. 47, 1067–1072 (2015).
Roberts, S. A. et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 45, 970–976 (2013).
Iyer, G. et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J. Clin. Oncol. 31, 3133–3140 (2013).
The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
van Oers, J. M. et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur. Urol. 55, 650–657 (2009).
Leiserson, M. D. et al. Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes. Nat. Genet. 47, 106–114 (2015).
Kolluri, K. K. et al. Loss of functional BAP1 augments sensitivity to TRAIL in cancer cells. eLife 7, e30224 (2018).
Miller, R. E. et al. Synthetic lethal targeting of ARID1A-mutant ovarian clear cell tumors with dasatinib. Mol. Cancer Ther. 15, 1472–1484 (2016).
Vandekerkhove, G. et al. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin. Cancer Res. 23, 6487–6497 (2017).
Togneri, F. S. et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur. J. Hum. Genet. 24, 1167–1174 (2016).
Wong, Y. N. S. et al. Urine-derived lymphocytes as a non-invasive measure of the bladder tumor immune microenvironment. J. Exp. Med. 215, 2748–2759 (2018).
Amin, M. B. et al. Update for the practicing pathologist: the International Consultation on Urologic Disease-European Association of Urology consultation on bladder cancer. Mod. Pathol. 28, 612–630 (2015).
Moschini, M. et al. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 14, 651 (2017).
von der Maase, H. et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 18, 3068–3077 (2000).
Sternberg, C. N. et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J. Urol. 133, 403–407 (1985).
Loehrer, P. J. Sr. et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J. Clin. Oncol. 10, 1066–1073 (1992).
Sternberg, C. N. et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer 64, 2448–2458 (1989).
von der Maase, H. et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 23, 4602–4608 (2005).
Sternberg, C. N. et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer protocol no. 30924. J. Clin. Oncol. 19, 2638–2646 (2001).
Bellmunt, J. et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 30, 1107–1113 (2012).
De Santis, M. et al. Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II—results of EORTC study 30986. J. Clin. Oncol. 27, 5634–5639 (2009).
Bellmunt, J. et al. A randomized phase II/III study of cabazitaxel versus vinflunine in metastatic or locally advanced transitional cell carcinoma of the urothelium (SECAVIN). Ann. Oncol. 28, 1517–1522 (2017).
Bellmunt, J. et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 27, 4454–4461 (2009).
Sonpavde, G. et al. Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol. 11, 861–870 (2010).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003).
Necchi, A. et al. Efficacy and safety of gemcitabine plus either taxane or carboplatin in the first-line setting of metastatic urothelial carcinoma: a systematic review and meta-analysis. Clin. Genitourin. Cancer 15, 23–30 (2017).
Necchi, A. et al. Cisplatin-based first-line therapy for advanced urothelial carcinoma after previous perioperative cisplatin-based therapy. Clin. Genitourin. Cancer 13, 178–184 (2015).
Sjodahl, G., Jackson, C. L., Bartlett, J. M., Siemens, D. R. & Berman, D. M. Molecular profiling in muscle-invasive bladder cancer: more than the sum of its parts. J. Pathol. 247, 563–573 (2019).
Seiler, R. et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur. Urol. 72, 544–554 (2017).
Felsenstein, K. M. & Theodorescu, D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat. Rev. Urol. 15, 92–111 (2018).
Plimack, E. R. et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur. Urol. 68, 959–967 (2015).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Ishida, Y., Agata, Y., Shibahara, K. & Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895 (1992).
Bardhan, K. et al. PD-1 inhibits the TCR signaling cascade by sequestering SHP-2 phosphatase, preventing its translocation to lipid rafts and facilitating Csk-mediated inhibitory phosphorylation of Lck [abstract]. J. Immunol. 196 (Suppl. 1), 128.15 (2016).
Nakanishi, J. et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 56, 1173–1182 (2007).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Fessas, P., Lee, H., Ikemizu, S. & Janowitz, T. A molecular and preclinical comparison of the PD-1-targeted T cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 44, 136–140 (2017).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017).
Sharma, P. et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 17, 1590–1598 (2016).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017).
Tan, S. et al. Distinct PD-L1 binding characteristics of therapeutic monoclonal antibody durvalumab. Protein Cell 9, 135–139 (2018).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Powles, T. et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) [abstract]. J. Clin. Oncol. 32 (Suppl. 5), 5011 (2014).
Powles, T. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391, 748–757 (2018).
Massard, C. et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 34, 3119–3125 (2016).
Powles, T. et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 3, e172411 (2017).
Apolo, A. B. et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J. Clin. Oncol. 35, 2117–2124 (2017).
Patel, M. R. et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 19, 51–64 (2018).
Powles, T. & Morrison, L. Biomarker challenges for immune checkpoint inhibitors in urothelial carcinoma. Nat. Rev. Urol. 15, 585–587 (2018).
Plimack, E. R. et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 18, 212–220 (2017).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Mariathasan, S. et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Robbins, P. F. et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752 (2013).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
van Rooij, N. et al. Tumor exome analysis reveals neoantigen-specific T cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–442 (2013).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Teo, M. Y. et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 36, 1685–1694 (2018).
van der Post, R. S. et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J. Med. Genet. 47, 464–470 (2010).
Akhurst, R. J. & Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811 (2012).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Pond, G. R. et al. A nomogram including baseline prognostic factors to estimate the activity of second-line therapy for advanced urothelial carcinoma. BJU Int. 113, E137–E143 (2014).
Pond, G. R. et al. New 6-factor prognostic model for patients (pts) with advanced urothelial carcinoma (UC) receiving post-platinum atezolizumab [abstract]. J. Clin. Oncol. 36 (Suppl. 6), 413 (2018).
Garcia, J. A. & Danielpour, D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol. Cancer Ther. 7, 1347–1354 (2008).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
Averous, J. & Proud, C. G. When translation meets transformation: the mTOR story. Oncogene 25, 6423–6435 (2006).
Mansure, J. J. et al. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol. Ther. 8, 2339–2347 (2009).
Milowsky, M. I. et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 112, 462–470 (2013).
Wulfing, C. et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 115, 2881–2890 (2009).
Powles, T. et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J. Clin. Oncol. 35, 48–55 (2017).
Turner, N. & Grose, R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer 10, 116–129 (2010).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1 (2013).
Iyer, G. & Milowsky, M. I. Fibroblast growth factor receptor-3 in urothelial tumorigenesis. Urol. Oncol. 31, 303–311 (2013).
Milowsky, M. I. et al. Phase 2 trial of dovitinib in patients with progressive FGFR3-mutated or FGFR3 wild-type advanced urothelial carcinoma. Eur. J. Cancer 50, 3145–3152 (2014).
Nogova, L. et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J. Clin. Oncol. 35, 157–165 (2017).
Loriot, Y. et al. Erdafitinib (ERDA; JNJ-42756493), a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRa): phase 2 continuous versus intermittent dosing. J. Clin. Oncol. 36, 411–411 (2018).
Petrylak, D. P. et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 390, 2266–2277 (2017).
Wit, R. D. et al. Ramucirumab (RAM) exposure-response (ER) relationship in RANGE: a randomized phase III trial of RAM plus docetaxel (DOC) versus placebo (P) plus DOC in advanced platinum-refractory urothelial carcinoma (UC) patients (pts). J. Clin. Oncol. 37, 353–353 (2019).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
Sui, W. et al. Micropapillary bladder cancer: insights from the National Cancer Database. Bladder Cancer 2, 415–423 (2016).
Comperat, E. et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology 42, 650–654 (2010).
Meeks, J. J. et al. Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int. 111, E325–E330 (2013).
Rosenblatt, R. et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur. Urol. 61, 1229–1238 (2012).
Guo, C. C. et al. Gene expression profile of the clinically aggressive micropapillary variant of bladder cancer. Eur. Urol. 70, 611–620 (2016).
Sahin, A. A. et al. Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol. 35, 277–280 (1991).
Raspollini, M. R. et al. Plasmacytoid urothelial carcinoma of the urinary bladder: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of a case series. Hum. Pathol. 42, 1149–1158 (2011).
Kaimakliotis, H. Z. et al. Plasmacytoid bladder cancer: variant histology with aggressive behavior and a new mode of invasion along fascial planes. Urology 83, 1112–1116 (2014).
Li, Q. et al. The impact of plasmacytoid variant histology on the survival of patients with urothelial carcinoma of bladder after radical cystectomy. Eur. Urol. Focus 5, 104–108 (2017).
Dayyani, F. et al. Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinomatosis. J. Urol. 189, 1656–1661 (2013).
Wang, J., Gillaspie, C., Kunadharaju, R., Talmon, G. A. & Enke, C. Sarcomatoid urothelial carcinoma: a single cancer center experience. World J. Oncol. 2, 175–180 (2011).
Sung, M. T. et al. Histogenesis of sarcomatoid urothelial carcinoma of the urinary bladder: evidence for a common clonal origin with divergent differentiation. J. Pathol. 211, 420–430 (2007).
Froehner, M., Gaertner, H. J., Manseck, A. & Wirth, M. P. Durable complete remission of metastatic sarcomatoid carcinoma of the bladder with cisplatin and gemcitabine in an 80-year-old man. Urology 58, 799 (2001).
Powell, M. A. et al. Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. J. Clin. Oncol. 28, 2727–2731 (2010).
Shokeir, A. A. Squamous cell carcinoma of the bladder: pathology, diagnosis and treatment. BJU Int. 93, 216–220 (2004).
Royce, T. J. et al. Clinical characteristics and outcomes of nonurothelial cell carcinoma of the bladder: results from the National Cancer Data Base. Urol. Oncol. 36, 78.e1–78.e12 (2018).
Mostafa, M. H., Sheweita, S. A. & O’Connor, P. J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 12, 97–111 (1999).
Willis, D. & Kamat, A. M. Nonurothelial bladder cancer and rare variant histologies. Hematol. Oncol. Clin. North Am. 29, 237–252 (2015).
Abdollah, F. et al. Survival after radical cystectomy of non-bilharzial squamous cell carcinoma versus urothelial carcinoma: a competing-risks analysis. BJU Int 109, 564–569 (2012).
Kassouf, W. et al. Outcome and patterns of recurrence of nonbilharzial pure squamous cell carcinoma of the bladder: a contemporary review of The University of Texas M D Anderson Cancer Center experience. Cancer 110, 764–769 (2007).
Kastritis, E. et al. The outcome of patients with advanced pure squamous or mixed squamous and transitional urothelial carcinomas following platinum-based chemotherapy. Anticancer Res. 26, 3865–3869 (2006).
Khaled, H. M., Hamza, M. R., Mansour, O., Gaafar, R. & Zaghloul, M. S. A phase II study of gemcitabine plus cisplatin chemotherapy in advanced bilharzial bladder carcinoma. Eur. J. Cancer 36 (Suppl. 2), 34–37 (2000).
Samlowski, W. E. et al. Evaluation of the combination of docetaxel/carboplatin in patients with metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN): a Southwest Oncology Group phase II study. Cancer Invest. 25, 182–188 (2007).
Shepherd, F. A. et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 18, 2095–2103 (2000).
Galsky, M. D. et al. Prospective trial of ifosfamide, paclitaxel, and cisplatin in patients with advanced non-transitional cell carcinoma of the urothelial tract. Urology 69, 255–259 (2007).
Molitor, M. et al. Comparison of structural genetics of non-schistosoma-associated squamous cell carcinoma of the urinary bladder. Int. J. Clin. Exp. Pathol. 8, 8143–8158 (2015).
Hovelson, D. H. et al. Targeted DNA and RNA sequencing of paired urothelial and squamous bladder cancers to reveal discordant genomic and transcriptomic events and unique therapeutic opportunities. J. Clin. Oncol. 35, 296–296 (2017).
Dotto, G. P. & Rustgi, A. K. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell 29, 622–637 (2016).
Abrahams, N. A., Moran, C., Reyes, A. O., Siefker-Radtke, A. & Ayala, A. G. Small cell carcinoma of the bladder: a contemporary clinicopathological study of 51 cases. Histopathology 46, 57–63 (2005).
Koay, E. J., Teh, B. S., Paulino, A. C. & Butler, E. B. A. Surveillance, epidemiology, and end results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer 117, 5325–5333 (2011).
Choong, N. W., Quevedo, J. F. & Kaur, J. S. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer 103, 1172–1178 (2005).
Lohrisch, C., Murray, N., Pickles, T. & Sullivan, L. Small cell carcinoma of the bladder: long term outcome with integrated chemoradiation. Cancer 86, 2346–2352 (1999).
Siefker-Radtke, A. O. et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J. Urol. 172, 481–484 (2004).
Pakkala, S. & Owonikoko, T. K. Immune checkpoint inhibitors in small cell lung cancer. J. Thorac Dis. 10, S460–S467 (2018).
Comperat, E., Varinot, J., Moroch, J., Eymerit-Morin, C. & Brimo, F. A practical guide to bladder cancer pathology. Nat. Rev. Urol. 15, 143–154 (2018).
Lughezzani, G. et al. Adenocarcinoma versus urothelial carcinoma of the urinary bladder: comparison between pathologic stage at radical cystectomy and cancer-specific mortality. Urology 75, 376–381 (2010).
Wright, J. L., Porter, M. P., Li, C. I., Lange, P. H. & Lin, D. W. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer 107, 721–728 (2006).
Szarvas, T. et al. Clinical, prognostic, and therapeutic aspects of urachal carcinoma—a comprehensive review with meta-analysis of 1,010 cases. Urol. Oncol. 34, 388–398 (2016).
Roy, S. et al. Next-generation sequencing-based molecular characterization of primary urinary bladder adenocarcinoma. Mod. Pathol. 30, 1133–1143 (2017).
Collazo-Lorduy, A. et al. Urachal carcinoma shares genomic alterations with colorectal carcinoma and may respond to epidermal growth factor inhibition. Eur. Urol. 70, 771–775 (2016).
Gopalan, A. et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am. J. Surg. Pathol. 33, 659–668 (2009).
Siefker-Radtke, A. Urachal adenocarcinoma: a clinician’s guide for treatment. Semin. Oncol. 39, 619–624 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00082706 (2018).
Loh, K. P. et al. Targeted therapy based on tumor genomic analyses in metastatic urachal carcinoma. Clin. Genitourin. Cancer 14, e449–452 (2016).
Wang, J. Primary sarcoma and sarcomatoid carcinoma of the urinary bladder: comparison of clinical features, treatment, and survival [abstract]. J. Clin. Oncol. 34 (Suppl. 2), 381 (2016).
Spiess, P. E. et al. Review of the M. D. Anderson experience in the treatment of bladder sarcoma. Urol. Oncol. 25, 38–45 (2007).
Linch, M., Miah, A. B., Thway, K., Judson, I. R. & Benson, C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat. Rev. Clin. Oncol. 11, 187–202 (2014).
Judson, I. et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 15, 415–423 (2014).
Seddon, B. et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 18, 1397–1410 (2017).
Mai, K. T., Bateman, J., Djordjevic, B., Flood, T. A. & Belanger, E. C. Clear cell urothelial carcinoma. Int. J. Surg. Pathol. 25, 18–25 (2017).
Oliva, E., Amin, M. B., Jimenez, R. & Young, R. H. Clear cell carcinoma of the urinary bladder: a report and comparison of four tumors of mullerian origin and nine of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am. J. Surg. Pathol. 26, 190–197 (2002).
Teo, M. Y. & Rosenberg, J. E. EMA and FDA prune the checkpoint inhibitor treatment landscape. Nat. Rev. Urol. 15, 596–597 (2018).
Gourd, E. EMA restricts use of anti-PD-1 drugs for bladder cancer. Lancet Oncol. 19, e341 (2018).
Balar, A. V. et al. Abstract CT112: durvalumab + tremelimumab in patients with metastatic urothelial cancer [abstract]. Cancer Res. 78 (Suppl. 13), CT112 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02516241 (2019).
[No authors listed]. Companies scaling back IDO1 inhibitor trials. Cancer Discov. 8, OF5 (2018).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02853305 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02807636 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02546661 (2019).
Peyton, C. C. et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 4, 1535–1542 (2018).
Powles, T. et al. A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS) [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 4506 (2018).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
Strauss, J. et al. Safety and activity of M7824, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with HPV associated cancers [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 3007 (2018).
Challita-Eid, P. M. et al. Enfortumab vedotin antibody–drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
Rosenberg, J. E. et al. EV-201 study: a single-arm, open-label, multicenter study of enfortumab vedotin for treatment of patients with locally advanced or metastatic urothelial cancer who previously received immune checkpoint inhibitor therapy. J. Clin. Oncol. 36 (Suppl. 15), TPS4590 (2018).
US Food and Drug Administration. FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma (updated 12 Apr 2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03474107 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03390504 (2019).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Coley, W. B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 262, 3–11 (1991).
Bulkley, G. B., Cohen, M. H., Banks, P. M., Char, D. H. & Ketcham, A. S. Long-term spontaneous regression of malignant melanoma with visceral metastases. Report of a case with immunologic profile. Cancer 36, 485–494 (1975).
Weber, J. et al. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2’-deoxycytidine. Cancer Res. 54, 1766–1771 (1994).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Simons, M., Gordon, E. & Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 17, 611–625 (2016).
Moch, H., Humphrey, P. A., Ulbright, T. M. & Reuter, V. E. WHO Classification of Tumours of the Urinary System and Male Genital Organs 4th edn (International Agency for Research on Cancer, Lyon, 2016).
Centre for Evidence-Based Medicine. Oxford Centre for Evidence-based Medicine – levels of evidence (March 2009). CEBM https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009 (2009).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Petrylak, D. P. et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer. JAMA Oncol. 4, 537 (2018).
Acknowledgements
M.L. and A.F. are supported by the National Institute for Health Research and the University College London Hospitals Biomedical Research Centre (no grant numbers apply). M.L. receives funding from Cancer Research UK, the Rosetrees Trust and a Bristol-Myers Squibb II-ON grant (no grant numbers apply).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussion of the article contents, wrote the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.L. has received research funding from Bristol-Myers Squibb, AstraZeneca/MedImmune and Astellas, and has received consulting fees from Janssen Pharmaceuticals. T.P. receives research funding from AstraZeneca/MedImmune, Genentech/Roche and Novartis, and consulting fees from Genentech/Roche, Pfizer, GlaxoSmithKline and Merck. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks G. Netto, I. Cunha, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Non-urothelial carcinomas
-
The minority of urothelial carcinomas that arise from cells other than urothelial cells, most commonly pure squamous cell carcinomas or pure adenocarcinomas.
- Conventional urothelial carcinoma
-
The most common type of bladder carcinoma, arising from urothelial cells lining the bladder and urinary tract, also known as the transitional epithelium.
- Urothelial carcinoma with divergent differentiation
-
Also known as variant urothelial carcinoma. A urothelial carcinoma with varying amounts of differentiation to other histological entities, of which 13 are currently recognized, including sarcomatoid and squamous differentiation.
- Luminal
-
In gene expression studies, ‘luminal’ describes a group of bladder cancers expressing epithelial markers.
- Basal
-
In gene expression studies, ‘basal’ describes a group of bladder cancers lacking epithelial markers but expressing markers of mesenchymal or sarcomatoid differentiation.
- Liquid biopsies
-
The use of circulating (dynamic) tumour-derived nucleic acids to inform tumour-specific somatic mutations.
- T cell exhaustion
-
A state of T cell dysfunction that exists in many chronic diseases and cancer due to prolonged antigen stimulation; defined by expression of inhibitory receptors and loss of effector function in T cells.
- Lynch syndrome
-
Also known as hereditary non-polyposis colorectal cancer. An inherited autosomal dominant condition that increases the risk of certain solid tumours such as colorectal and endometrial cancer; caused by mutations in DNA mismatch repair genes, such as MLH1 and MSH2.
- Keratinizing squamous metaplasia
-
A precancerous condition of squamous cells.
- Bilharzial-associated bladder cancer
-
Bladder cancer arising following chronic infection with schistosomiasis (infection also known as snail fever and bilharzia).
- Leiomyosarcoma
-
A malignant tumour arising from smooth muscle.
- Angiosarcoma
-
A malignant tumour arising from blood vessels.
Rights and permissions
About this article
Cite this article
Alifrangis, C., McGovern, U., Freeman, A. et al. Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol 16, 465–483 (2019). https://doi.org/10.1038/s41585-019-0208-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-019-0208-0
This article is cited by
-
Molecular classification of high-grade, muscle-invasive urothelial carcinomas and the relationship with CTLA-4 and PD-L1 expression
Surgical and Experimental Pathology (2023)
-
Immune inactivation by VISTA predicts clinical outcome and therapeutic benefit in muscle-invasive bladder cancer
BMC Cancer (2023)
-
Apoptosis related genes mediated molecular subtypes depict the hallmarks of the tumor microenvironment and guide immunotherapy in bladder cancer
BMC Medical Genomics (2023)
-
Exosome, the glass slipper for Cinderella of cancer—bladder cancer?
Journal of Nanobiotechnology (2023)
-
Integrated multi-omics analyses reveal Jorunnamycin A as a novel suppressor for muscle-invasive bladder cancer by targeting FASN and TOP1
Journal of Translational Medicine (2023)