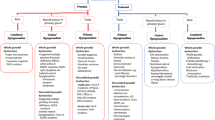
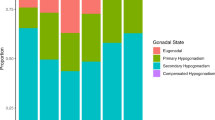
Abstract
The hypothalamic–pituitary–gonadal axis is of relevance in many processes related to the development, maturation and ageing of the male. Through this axis, a cascade of coordinated activities is carried out leading to sustained testicular endocrine function, with gonadal testosterone production, as well as exocrine function, with spermatogenesis. Conditions impairing the hypothalamic–pituitary–gonadal axis during paediatric or pubertal life may result in delayed puberty. Late-onset hypogonadism is a clinical condition in the ageing male combining low concentrations of circulating testosterone and specific symptoms associated with impaired hormone production. Testosterone therapy for congenital forms of hypogonadism must be lifelong, whereas testosterone treatment of late-onset hypogonadism remains a matter of debate because of unclear indications for replacement, uncertain efficacy and potential risks. This Primer focuses on a reappraisal of the physiological role of testosterone, with emphasis on the critical interpretation of the hypogonadal conditions throughout the lifespan of the male individual, with the exception of hypogonadal states resulting from congenital disorders of sex development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Corradi, P. F., Corradi, R. B. & Greene, L. W. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol. Clin. North Am. 43, 151–162 (2016). This manuscript comprehensively describes the complex physiology of the male HPG axis.
Flück, C. E. et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am. J. Hum. Genet. 89, 201–218 (2011).
Ross, A. & Bhasin, S. Hypogonadism: its prevalence and diagnosis. Urol. Clin. North Am. 43, 163–176 (2016).
Rey, R. A. et al. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology 1, 3–16 (2013). This work provides a classification of male hypogonadism, explaining the pathophysiology and specific diagnostic procedures needed according to the age of establishment of the disorder, from fetal life to adulthood.
Rastrelli, G., Vignozzi, L. & Maggi, M. Different medications for hypogonadotropic hypogonadism. Endocr. Dev. 30, 60–78 (2016).
Lippincott, M. F. et al. Kisspeptin responsiveness signals emergence of reproductive endocrine activity: implications for human puberty. J. Clin. Endocrinol. Metab. 101, 3061–3069 (2016).
Hughes, I. A., Nihoul-Fékété, C., Thomas, B. & Cohen-Kettenis, P. T. Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. 21, 351–365 (2007).
Maggi, M. & Buvat, J. Standard operating procedures: pubertas tarda/delayed puberty—male. J. Sex. Med. 10, 285–293 (2013).
Sedlmeyer, I. L. Delayed puberty: analysis of a large case series from an academic center. J. Clin. Endocrinol. Metab. 87, 1613–1620 (2002).
Lawaetz, J. G. et al. Evaluation of 451 Danish boys with delayed puberty: diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. J. Clin. Endocrinol. Metab. 100, 1376–1385 (2015).
Varimo, T., Miettinen, P. J., Känsäkoski, J., Raivio, T. & Hero, M. Congenital hypogonadotropic hypogonadism, functional hypogonadotropism or constitutional delay of growth and puberty? An analysis of a large patient series from a single tertiary center. Hum. Reprod. 32, 147–153 (2016).
Abitbol, L., Zborovski, S. & Palmert, M. R. Evaluation of delayed puberty: what diagnostic tests should be performed in the seemingly otherwise well adolescent? Arch. Dis. Child. 101, 767–771 (2016).
Piel, F. B., Steinberg, M. H. & Rees, D. C. Sickle cell disease. N. Engl. J. Med. 376, 1561–1573 (2017).
Kupczyk, M. & Wenzel, S. US and European severe asthma cohorts: what can they teach us about severe asthma? J. Intern. Med. 272, 121–132 (2012).
Cosnes, J., Gower–Rousseau, C., Seksik, P. & Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140, 1785–1794 (2011).
Benchimol, E. I. et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm. Bowel Dis. 17, 423–439 (2011).
Bonomi, M. et al. Characteristics of a nationwide cohort of patients presenting with isolated hypogonadotropic hypogonadism (IHH). Eur. J. Endocrinol. 178, 23–32 (2018).
Franco, B. et al. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353, 529–536 (1991).
Guyot-Goubin, A. et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr. Blood Cancer 51, 71–75 (2008).
Bakhsheshian, J. et al. Risk factors associated with the surgical management of craniopharyngiomas in pediatric patients: analysis of 1961 patients from a national registry database. Neurosurg. Focus 41, E8 (2016).
Bonomi, M. et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J. Endocrinol. Invest. 40, 123–134 (2016).
Kanakis, G. A. & Nieschlag, E. Klinefelter syndrome: more than hypogonadism. Metabolism 86, 135–144 (2018).
Aksglaede, L. et al. 47,XXY Klinefelter syndrome: Clinical characteristics and age-specific recommendations for medical management. Am. J. Med. Genet. C 163, 55–63 (2013).
Bojesen, A., Juul, S. & Gravholt, C. H. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J. Clin. Endocrinol. Metab. 88, 622–626 (2003).
Gravholt, C. H. et al. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr. Rev. 39, 389–423 (2018).
Leader, A., Lishner, M., Michaeli, J. & Revel, A. Fertility considerations and preservation in haemato-oncology patients undergoing treatment. Br. J. Haematol. 153, 291–308 (2011).
Harman, S. M. et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J. Clin. Endocrinol. Metab. 86, 724–731 (2001).
Wu, F. C. W. et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 363, 123–135 (2010). This study defines the diagnostic criteria and prevalence of symptomatic LOH in the European male population.
Tajar, A. et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J. Clin. Endocrinol. Metab. 95, 1810–1818 (2010).
Eendebak, R. J. A. H. et al. Elevated luteinizing hormone despite normal testosterone levels in older men-natural history, risk factors and clinical features. Clin. Endocrinol. 88, 479–490 (2018).
Grossmann, M. & Matsumoto, A. M. A. Perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J. Clin. Endocrinol. Metab. 102, 1067–1075 (2017). This study launches the new concept of functional hypogonadism.
Maseroli, E. et al. Prevalence of endocrine and metabolic disorders in subjects with erectile dysfunction: a comparative study. J. Sex. Med. 12, 956–965 (2015).
Kuiri-Hänninen, T., Sankilampi, U. & Dunkel, L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm. Res. Paediatr. 82, 73–80 (2014). This paper reviews the physiology and pathophysiology of disorders occurring during the postnatal activation of the gonadal axis.
Bergadá, I. et al. Time course of the serum gonadotropin surge, inhibins, and anti-Müllerian hormone in normal newborn males during the first month of life. J. Clin. Endocrinol. Metab. 91, 4092–4098 (2006).
Grinspon, R. P. et al. Male central precocious puberty: serum profile of anti-Müllerian hormone and inhibin B before, during, and after treatment with GnRH analogue. Int. J. Endocrinol. 2013, 823064 (2013).
Dunkel, L. & Quinton, R. Transition in endocrinology: induction of puberty. Eur. J. Endocrinol. 170, R229–R239 (2014).
Mouritsen, A. et al. The pubertal transition in 179 healthy Danish children: associations between pubarche, adrenarche, gonadarche, and body composition. Eur. J. Endocrinol. 168, 129–136 (2013).
Nathan, B. M. & Palmert, M. R. Regulation and disorders of pubertal timing. Endocrinol. Metab. Clin. North Am. 34, 617–641 (2005). This paper describes the physiology and pathophysiology of disorders occurring during the pubertal timing.
Marshall, W. A. & Tanner, J. M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 (1969).
Marshall, W. A. & Tanner, J. M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 (1970). This manuscript is still a milestone in the field.
Grinspon, R. P. et al. Spreading the clinical window for diagnosing fetal-onset hypogonadism in boys. Front. Endocrinol. 5, 51 (2014).
Grinspon, R. P., Urrutia, M. & Rey, R. A. Male central hypogonadism in paediatrics — the relevance of follicle-stimulating hormone and Sertoli cell markers. Eur. Endocrinol. 14, 67 (2018).
Andersson, A. M. & Skakkebaek, N. E. Serum inhibin B levels during male childhood and puberty. Mol. Cell. Endocrinol. 180, 103–107 (2001).
Neto, F. T. L., Bach, P. V., Najari, B. B., Li, P. S. & Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 59, 10–26 (2016).
Khera, M. et al. Diagnosis and treatment of testosterone deficiency: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J. Sex. Med. 13, 1787–1804 (2016). This manuscript mostly deals with the paramount role of decreasing testosterone levels in terms of male sexual function impairment according to both the literature and the opinion of a panel of experts in the field of sexual medicine.
Swiecicka, A. et al. Reproductive hormone levels predict changes in frailty status in community-dwelling older men: European Male Ageing Study Prospective Data. J. Clin. Endocrinol. Metab. 103, 701–709 (2017).
Yeap, B. B. Hormonal changes and their impact on cognition and mental health of ageing men. Maturitas 79, 227–235 (2014).
Hsu, B. et al. Temporal trend in androgen status and androgen-sensitive outcomes in older men. J. Clin. Endocrinol. Metab. 101, 1836–1846 (2016).
Holmboe, S. A. et al. Individual testosterone decline and future mortality risk in men. Eur. J. Endocrinol. 178, 121–128 (2018).
Travison, T. G. et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J. Clin. Endocrinol. Metab. 102, 1161–1173 (2017). This study is the first to detail reference ranges for serum testosterone concentrations throughout the male age span.
Wang, Y., Chen, F., Ye, L., Zirkin, B. & Chen, H. Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction 154, R111–R122 (2017).
Neaves, W. B., Johnson, L., Proter, J. C., Parker, C. R. Jr & Petty, C. S. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J. Clin. Endocrinol. Metab. 59, 756–763 (1984).
Simoni, M. & Huhtaniemi, I. (eds) Endocrinology of the Testis and Male Reproduction (Springer International Publishing, 2017).
Whitcomb, R. W. & Crowley, W. F. Hypogonadotropic hypogonadism: gonadotropin-releasing hormone therapy. Curr. Ther. Endocrinol. Metab. 6, 353–355 (1997).
Spratt, D. I. et al. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J. Clin. Endocrinol. Metab. 64, 283–291 (1987).
Raivio, T. et al. Reversal of idiopathic hypogonadotropic hypogonadism. N. Engl. J. Med. 357, 863–873 (2007).
Sidhoum, V. F. et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J. Clin. Endocrinol. Metab. 99, 861–870 (2014). This study is of paramount relevance in the discussion of the potential reversal of idiopathic hypogonadotropic hypogonadism.
Pierzchlewska, M. M., Robaczyk, M. G. & Vogel, I. Induction of puberty with human chorionic gonadotropin (hCG) followed by reversal of hypogonadotropic hypogonadism in Kallmann syndrome. Endokrynol. Pol. 68, 692–696 (2015).
Santhakumar, A., Balasubramanian, R., Miller, M. & Quinton, R. Reversal of isolated hypogonadotropic hypogonadism: long-term integrity of hypothalamo-pituitary-testicular axis in two men is dependent on intermittent androgen exposure. Clin. Endocrinol. 81, 473–476 (2013).
Finkelstein, J. S. et al. Pulsatile gonadotropin secretion after discontinuation of long term gonadotropin-releasing hormone (GnRH) administration in a subset of GnRH-deficient men. J. Clin. Endocrinol. Metab. 69, 377–385 (1989).
Pitteloud, N. et al. The fertile eunuch variant of idiopathic hypogonadotropic hypogonadism: spontaneous reversal associated with a homozygous mutation in the gonadotropin-releasing hormone receptor 1. J. Clin. Endocrinol. Metab. 86, 2470–2475 (2001).
Pitteloud, N. et al. Reversible Kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J. Clin. Endocrinol. Metab. 90, 1317–1322 (2005).
Laitinen, E.-M. et al. Reversible congenital hypogonadotropic hypogonadism in patients with CHD7, FGFR1 or GNRHR mutations. PLOS ONE 7, e39450 (2012).
Waldstreicher, J. et al. The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. J. Clin. Endocrinol. Metab. 81, 4388–4395 (1996).
Dodé, C. et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat. Genet. 33, 463–465 (2003).
Ayari, B. & Soussi-Yanicostas, N. FGFR1 and anosmin-1 underlying genetically distinct forms of Kallmann syndrome are co-expressed and interact in olfactory bulbs. Dev. Genes Evol. 217, 169–175 (2007).
Topaloglu, A. K. et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 366, 629–635 (2012).
Silveira, L. G., Latronico, A. C. & Seminara, S. B. Kisspeptin and clinical disorders. Adv. Exp. Med. Biol. 784, 187–199 (2013).
Goodman, R. L. et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148, 5752–5760 (2007).
Ramaswamy, S. et al. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151, 4494–4503 (2010).
Gianetti, E. et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J. Clin. Endocrinol. Metab. 95, 2857–2867 (2010).
Zhu, J. et al. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 100, E646–E654 (2015).
Perry, J. R. et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 514, 92–97 (2014).
Day, F. R. et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 49, 834–841 (2017).
Shi, Z., Araujo, A. B., Martin, S., O’Loughlin, P. & Wittert, G. A. Longitudinal changes in testosterone over five years in community-dwelling men. J. Clin. Endocrinol. Metab. 98, 3289–3297 (2013).
Wu, F. C. W. et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J. Clin. Endocrinol. Metab. 93, 2737–2745 (2008).
Feldman, H. A. et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 87, 589–598 (2002).
Rastrelli, G. et al. Symptomatic androgen deficiency develops only when both total and free testosterone decline in obese men who may have incident biochemical secondary hypogonadism: Prospective results from the EMAS. Clin. Endocrinol. 89, 459–469 (2018).
Perheentupa, A. & Huhtaniemi, I. Aging of the human ovary and testis. Mol. Cell. Endocrinol. 299, 2–13 (2009).
Camacho, E. M. et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur. J. Endocrinol. 168, 445–455 (2013).
Corona, G. et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur. J. Endocrinol. 168, 829–843 (2013). This study reports the findings of a systematic review and meta-analysis that outline that weight gain suppresses and weight loss increases circulating testosterone levels.
Corona, G., Vignozzi, L., Sforza, A., Mannucci, E. & Maggi, M. Obesity and late-onset hypogonadism. Mol. Cell. Endocrinol. 418, 120–133 (2015).
Antonio, L. et al. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J. Clin. Endocrinol. Metab. 101, 2647–2657 (2016).
Tsatsanis, C. et al. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Horm. Athens Greece 14, 549–562 (2015).
Brüning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Tena-Sempere, M. Interaction between energy homeostasis and reproduction: central effects of leptin and ghrelin on the reproductive axis. Horm. Metab. Res. 45, 919–927 (2013).
Luukkaa, V. et al. Inverse correlation between serum testosterone and leptin in men. J. Clin. Endocrinol. Metab. 83, 3243–3246 (1998).
Isidori, A. M. et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 84, 3673–3680 (1999).
Landry, D., Cloutier, F. & Martin, L. J. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod. Biol. 13, 1–14 (2013).
Banks, W. A. et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53, 1253–1260 (2004).
Ye, Z., Liu, G., Guo, J. & Su, Z. Hypothalamic endoplasmic reticulum stress as a key mediator of obesity-induced leptin resistance. Obes. Rev. 19, 770–785 (2018).
Pittas, A. G., Joseph, N. A. & Greenberg, A. S. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab. 89, 447–452 (2004).
Veldhuis, J., Yang, R., Roelfsema, F. & Takahashi, P. Proinflammatory cytokine infusion attenuates LH\textquotesingles feedforward on testosterone secretion: modulation by age. J. Clin. Endocrinol. Metab. 101, 539–549 (2016).
Pagotto, U., Marsicano, G., Cota, D., Lutz, B. & Pasquali, R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 27, 73–100 (2006).
Porte, D., Baskin, D. G. & Schwartz, M. W. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 54, 1264–1276 (2005).
George, J. T., Millar, R. P. & Anderson, R. A. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 91, 302–307 (2010).
Aarts, E. et al. Gonadal status and outcome of bariatric surgery in obese men. Clin. Endocrinol. 81, 378–386 (2013).
Pellitero, S. et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes. Surg. 22, 1835–1842 (2012).
Chosich, J. et al. Acute recapitulation of the hyperinsulinemia and hyperlipidemia characteristic of metabolic syndrome suppresses gonadotropins. Obesity 25, 553–560 (2017).
Grinspon, R. P. et al. Gonadotrophin secretion pattern in anorchid boys from birth to pubertal age: pathophysiological aspects and diagnostic usefulness. Clin. Endocrinol. 76, 698–705 (2012).
Grinspon, R. P. & Rey, R. A. Anti-müllerian hormone and sertoli cell function in paediatric male hypogonadism. Horm. Res. Paediatr. 73, 81–92 (2010).
Grinspon, R. P. et al. Early onset of primary hypogonadism revealed by serum anti-Müllerian hormone determination during infancy and childhood in trisomy 21. Int. J. Androl. 34, e487–e498 (2011).
Bastida, M. G. et al. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin. Endocrinol. 67, 863–870 (2007).
Boehm, U. et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 11, 547–564 (2015). This manuscript reports the findings of an expert consensus regarding the pathogenesis, diagnosis and treatment of congenital hypogonadotropic hypogonadism.
Palmert, M. R. & Dunkel, L. Clinical practice. Delayed puberty. N. Engl. J. Med. 366, 443–453 (2012).
Grinspon, R. P. et al. Basal follicle-stimulating hormone and peak gonadotropin levels after gonadotropin-releasing hormone infusion show high diagnostic accuracy in boys with suspicion of hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 95, 2811–2818 (2010).
Coutant, R. et al. Baseline inhibin B and anti-Mullerian hormone measurements for diagnosis of hypogonadotropic hypogonadism (HH) in boys with delayed puberty. J. Clin. Endocrinol. Metab. 95, 5225–5232 (2010).
Sykiotis, G. P. et al. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J. Clin. Endocrinol. Metab. 95, 3019–3027 (2010).
Au, M. G., Crowley, W. F. & Buck, C. L. Genetic counseling for isolated GnRH deficiency. Mol. Cell. Endocrinol. 346, 102–109 (2011).
Stamou, M. I., Cox, K. H. & Crowley, W. F. Discovering genes essential to the hypothalamic regulation of human reproduction using a human disease model: adjusting to life in the “-omics” era. Endocr. Rev. 36, 603–621 (2015).
Bhasin, S. et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 95, 2536–2559 (2010).
Bhasin, S. et al. Testosterone therapy in men with hypogonadism: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 103, 1715–1744 (2018). This manuscript details the most updated clinical practice guidelines recently released by the Endocrine Society.
Hackett, G. et al. British Society for Sexual Medicine Guidelines on adult testosterone deficiency, with statements for UK practice. J. Sex. Med. 14, 1504–1523 (2017). This manuscript reports the most recent recommendations released by the BSSM on adult testosterone deficiency, even as a part of the real-life experience.
Petak, S. M. et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients–2002 update. Endocr. Pract. 8, 440–456 (2002). This paper is a pivotal manuscript in the field of diagnostic work-up and treatment of adult male patients with hypogonadism.
Jasuja, G. K., Bhasin, S., Reisman, J. I., Berlowitz, D. R. & Rose, A. J. Ascertainment of testosterone prescribing practices in the VA. Med. Care 53, 746–752 (2015).
Baillargeon, J., Kuo, Y.-F., Westra, J. R., Urban, R. J. & Goodwin, J. S. Testosterone prescribing in the United States, 2002–2016. JAMA 320, 200 (2018).
Snyder, P. J. et al. Effects of testosterone treatment in older men. N. Engl. J. Med. 374, 611–624 (2016).
Snyder, P. J. et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J. Clin. Endocrinol. Metab. 84, 1966–1972 (1999).
Walther, A., Breidenstein, J. & Miller, R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiatry 76, 31 (2019).
European Association of Urology. EAU Guidelines on male hypogonadism. EAU https://uroweb.org/guideline/male-hypogonadism/ (2018).
Taylor, A. E., Keevil, B. & Huhtaniemi, I. T. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 173, D1–D12 (2015).
Basaria, S. et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels. JAMA 314, 570 (2015).
Glass, A. R., Swerdloff, R. S., Bray, G. A., Dahms, W. T. & Atkinson, R. L. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J. Clin. Endocrinol. Metab. 45, 1211–1219 (1977).
Stanworth, R. D. & Jones, T. H. Testosterone for the aging male; current evidence and recommended practice. Clin. Interv. Aging 3, 25–44 (2008).
Vermeulen, A., Verdonck, L. & Kaufman, J. M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 84, 3666–3672 (1999). This manuscript describes the most widely used formula for determining the calculated free testosterone value.
Giton, F. Serum bioavailable testosterone: assayed or calculated? Clin. Chem. 52, 474–481 (2006).
Hackbarth, J. S., Hoyne, J. B., Grebe, S. K. & Singh, R. J. Accuracy of calculated free testosterone differs between equations and depends on gender and SHBG concentration. Steroids 76, 48–55 (2011).
Boeri, L. et al. Does calculated free testosterone overcome total testosterone in protecting from sexual symptom impairment? Findings of a cross-sectional study. J. Sex. Med. 14, 1549–1557 (2017).
Andrade-Rocha, F. T. Semen analysis in laboratory practice: an overview of routine tests. J. Clin. Lab. Anal. 17, 247–258 (2003).
Rhoden, E. L. et al. The value of pituitary magnetic resonance imaging in men with hypogonadism. J. Urol. 170, 795–798 (2003).
Molitch, M. E. Diagnosis and treatment of pituitary adenomas. JAMA 317, 516 (2017).
Chiloiro, S. et al. Diagnosis of endocrine disease: primary empty sella: a comprehensive review. Eur. J. Endocrinol. 177, R275–R285 (2017).
Corona, G., Rastrelli, G., Vignozzi, L. & Maggi, M. Emerging medication for the treatment of male hypogonadism. Expert Opin. Emerg. Drugs 17, 239–259 (2012).
Corona, G., Ratrelli, G. & Maggi, M. The pharmacotherapy of male hypogonadism besides androgens. Expert Opin. Pharmacother. 16, 369–387 (2014).
Rey, R. A. & Grinspon, R. P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. 25, 221–238 (2011).
Lee, P. A. et al. Global Disorders of Sex Development Update since 2006: perceptions, approach and care. Horm. Res. Paediatr. 85, 158–180 (2016).
Howard, S. & Dunkel, L. Sex steroid and gonadotropin treatment in male delayed puberty. Endocr. Dev. 29, 185–197 (2016).
Wei, C. & Crowne, E. C. Recent advances in the understanding and management of delayed puberty. Arch. Dis. Child. 101, 481–488 (2015). This manuscript summarizes recent advances regarding the neuroendocrine, genetic and environmental factors controlling pubertal development, with potential correlations in terms of delayed puberty pathophysiology.
Giri, D. et al. Testosterone therapy improves the first year height velocity in adolescent boys with constitutional delay of growth and puberty. Int. J. Endocrinol. Metab. 15, e42311 (2017).
Richman, R. A. & Kirsch, L. R. Testosterone treatment in adolescent boys with constitutional delay in growth and development. N. Engl. J. Med. 319, 1563–1567 (1988).
Spratt, D. I. et al. Subcutaneous injection of testosterone is an effective and preferred alternative to intramuscular injection: demonstration in female-to-male transgender patients. J. Clin. Endocrinol. Metab. 102, 2349–2355 (2017).
Chioma, L., Papucci, G., Fintini, D. & Cappa, M. Use of testosterone gel compared to intramuscular formulation for puberty induction in males with constitutional delay of growth and puberty: a preliminary study. J. Endocrinol. Invest. 41, 259–263 (2017).
Rogol, A. D. et al. A multicenter, open-label, observational study of testosterone gel (1%) in the treatment of adolescent boys with Klinefelter syndrome or anorchia. J. Adolesc. Health 54, 20–25 (2014).
Rogol, A. D., Tkachenko, N. & Bryson, N. NatestoTM, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology 4, 46–54 (2015).
Decourt, C. et al. A synthetic kisspeptin analog that triggers ovulation and advances puberty. Sci. Rep. 6, 26908 (2016).
Shulman, D. I., Francis, G. L., Palmert, M. R. & Eugster, E. A. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics 121, e975–e983 (2008).
Wit, J. M., Hero, M. & Nunez, S. B. Aromatase inhibitors in pediatrics. Nat. Rev. Endocrinol. 8, 135–147 (2011).
Varimo, T. et al. Letrozole versus testosterone for promotion of endogenous puberty in boys with constitutional delay of growth and puberty: a randomised controlled phase 3 trial. Lancet Child Adolesc. Health 3, 109–120 (2019).
Rohayem, J., Hauffa, B. P., Zacharin, M., Kliesch, S. & Zitzmann, M. Testicular growth and spermatogenesis: new goals for pubertal hormone replacement in boys with hypogonadotropic hypogonadism? -a multicentre prospective study of hCG/rFSH treatment outcomes during adolescence-. Clin. Endocrinol. 86, 75–87 (2016).
Gong, C., Liu, Y., Qin, M., Wu, D. & Wang, X. Pulsatile GnRH is superior to hCG in therapeutic efficacy in adolescent boys with hypogonadotropic hypogonadodism. J. Clin. Endocrinol. Metab. 100, 2793–2799 (2015).
Rastrelli, G., Corona, G., Mannucci, E. & Maggi, M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology 2, 794–808 (2014).
Aksglaede, L. & Juul, A. Therapy of endocrine disease: testicular function and fertility in men with Klinefelter syndrome: a review. Eur. J. Endocrinol. 168, R67–R76 (2013).
Forti, G., Corona, G., Vignozzi, L., Krausz, C. & Maggi, M. Klinefelter’s syndrome: a clinical and therapeutical update. Sex. Dev. 4, 249–258 (2010).
Corona, G. et al. Sexual dysfunction in subjects with Klinefelter’s syndrome. Int. J. Androl. 33, 574–580 (2009).
Vignozzi, L., Corona, G., Forti, G., Jannini, E. A. & Maggi, M. Clinical and therapeutic aspects of Klinefelter’s syndrome: sexual function. Mol. Hum. Reprod. 16, 418–424 (2010).
Corona, G. et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 23, 265–275 (2017). This manuscript reports novel findings of a meta-analysis devoted to defining positive sperm retrieval outcomes at surgery in men with Klinefelter syndrome.
Nahata, L. et al. Sperm retrieval in adolescents and young adults with Klinefelter syndrome: a prospective, pilot study. J. Pediatr. 170, 260–265 (2016).
Huhtaniemi, I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J. Androl. 16, 192 (2014).
Rastrelli, G. et al. Development of and recovery from secondary hypogonadism in aging men: prospective results from the EMAS. J. Clin. Endocrinol. Metab. 100, 3172–3182 (2015). This manuscripts reports the findings of the EMAS study, a prospective observational general population cohort survey, which outlines that obesity-related metabolic and lifestyle factors predispose older men to the development of secondary hypogonadism, which is frequently reversible with weight loss.
Kumagai, H. et al. Vigorous physical activity is associated with regular aerobic exercise-induced increased serum testosterone levels in overweight/obese men. Horm. Metab. Res. 50, 73–79 (2018).
Shao, N. et al. Short-term combined treatment with exenatide and metformin is superior to glimepiride combined metformin in improvement of serum testosterone levels in type 2 diabetic patients with obesity. Andrologia 50, e13039 (2018).
Ng Tang Fui, M., Hoermann, R., Zajac, J. D. & Grossmann, M. The effects of testosterone on body composition in obese men are not sustained after cessation of testosterone treatment. Clin. Endocrinol. 87, 336–343 (2017).
Brock, G. et al. Effect of testosterone solution 2% on testosterone concentration, sex drive and energy in hypogonadal men: results of a placebo controlled study. J. Urol. 195, 699–705 (2016).
Hackett, G. et al. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 118, 804–813 (2016).
Hackett, G. et al. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J. Sex. Med. 11, 840–856 (2014).
Jones, T. H. et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34, 828–837 (2011).
Tracz, M. J. et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J. Clin. Endocrinol. Metab. 91, 2011–2016 (2006).
Kenny, A. M. et al. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J. Am. Geriatr. Soc. 58, 1707–1714 (2010).
Snyder, P. J. et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone. JAMA Intern. Med. 177, 471 (2017).
Gennari, L. & Bilezikian, J. P. New and developing pharmacotherapy for osteoporosis in men. Expert Opin. Pharmacother. 19, 253–264 (2018).
Rochira, V., Antonio, L. & Vanderschueren, D. EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology 6, 272–285 (2018).
Bachman, E. et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A 69, 725–735 (2014).
Roy, C. N. et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern. Med. 177, 480 (2017).
Khera, M. et al. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 15, 14–21 (2011).
Schneider, G. et al. Depressive symptoms in men aged 50 years and older and their relationship to genetic androgen receptor polymorphism and sex hormone levels in three different samples. Am. J. Geriatr. Psychiatry 19, 274–283 (2011).
Schneider, G., Zitzmann, M., Gromoll, J., Ladwig, K. H. & Berger, K. The relation between sex hormone levels, the androgen receptor CAGn-polymorphism and depression and mortality in older men in a community study. Psychoneuroendocrinology 38, 2083–2090 (2013).
Resnick, S. M. et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 317, 717–727 (2017).
Bhasin, S. et al. Effect of testosterone replacement on measures of mobility in older men with mobility limitation and low testosterone concentrations: secondary analyses of the Testosterone Trials. Lancet Diabetes Endocrinol. 6, 879–890 (2018).
Holmegard, H. N., Nordestgaard, B. G., Schnohr, P., Tybjaerg-Hansen, A. & Benn, M. Endogenous sex hormones and risk of venous thromboembolism in women and men. J. Thromb. Haemost. 12, 297–305 (2014).
Baillargeon, J., Kuo, Y.-F., Fang, X. & Shahinian, V. B. Long-term exposure to testosterone therapy and the risk of high grade prostate cancer. J. Urol. 194, 1612–1616 (2015).
Dohle, G. R., Smit, M. & Weber, R. F. A. Androgens and male fertility. World J. Urol. 21, 341–345 (2003).
Soisson, V. et al. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C cohort study. Maturitas 75, 282–288 (2013).
Corona, G. et al. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J. Sex. Med. 15, 1260–1271 (2018).
Saad, F., Röhrig, G., Haehling, S. von & Traish, A. Testosterone deficiency and testosterone treatment in older men. Gerontology 63, 144–156 (2016).
Corona, G. et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J. Endocrinol. Invest. 39, 967–981 (2016).
Corona, G. et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur. J. Endocrinol. 174, R99–R116 (2016).
Corona, G., Rastrelli, G., Reisman, Y., Sforza, A. & Maggi, M. The safety of available treatments of male hypogonadism in organic and functional hypogonadism. Expert Opin. Drug Saf. 17, 277–292 (2018).
Etminan, M., Skeldon, S. C., Goldenberg, S. L., Carleton, B. & Brophy, J. M. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy 35, 72–78 (2015).
US Food & Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov https://www.fda.gov/Drugs/DrugSafety/ucm436259.htm (updated 26 Feb 2018).
Government of Canada Drug and Health Product Register. Summary safety review — testosterone replacement products — Health Canada. Health Canada https://hpr-rps.hres.ca/reg-content/summary-safety-review-detail.php?linkID=SSR00058 (2014).
Yeap, B. B. et al. Endocrine Society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med. J. Aust. 205, 173–178 (2016).
European Medicines Agency. Testosterone-containing medicines. Europa.eu https://www.ema.europa.eu/en/medicines/human/referrals/testosterone-containing-medicines (updated 8 Jan 2015).
Corona, G. et al. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J. Sex. Med. 15, 820–838 (2018).
Luo, S., Au Yeung, S. L., Zhao, J. V., Burgess, S. & Schooling, C. M. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK Biobank. BMJ 364, l476 (2019).
Coviello, A. D. et al. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J. Clin. Endocrinol. Metab. 93, 914–919 (2008).
Hoyos, C. M., Killick, R., Yee, B. J., Grunstein, R. R. & Liu, P. Y. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin. Endocrinol. 77, 599–607 (2012).
Corona, G. et al. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur. Urol. 72, 1000–1011 (2017). This manuscript is clinically relevant in saying that testosterone therapy significantly improves erectile function in men with more severe hypogonadism compared with those with milder testosterone deficiency.
Ponce, O. J. et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Endocrinol. Metab. 103, 1745–1754 (2018).
Kaufman, J. M., Lapauw, B., Mahmoud, A., T’Sjoen, G. & Huhtaniemi, I. T. Aging and the male reproductive system. Endocr. Rev. https://doi.org/10.1210/er.2018-00178 (2019).
Isidori, A. M. et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin. Endocrinol. 63, 381–394 (2005).
Hatzimouratidis, K. et al. Pharmacotherapy for erectile dysfunction: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J. Sex. Med. 13, 465–488 (2016).
Isidori, A. M. et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment—a systematic review. Eur. Urol. 65, 99–112 (2014). This manuscript describes the findings of a critical reappraisal of the role of testosterone in terms of erectile function physiology and pathophysiology.
Corona, G. et al. Testosterone supplementation and sexual function: a meta-analysis study. J. Sex. Med. 11, 1577–1592 (2014).
Belling, K. et al. Klinefelter syndrome comorbidities linked to increased X chromosome gene dosage and altered protein interactome activity. Hum. Mol. Genet. 26, 1219–1229 (2017).
D’Aurora, M. et al. Testis transcriptome modulation in Klinefelter patients with hypospermatogenesis. Sci. Rep. 7, 45729 (2017).
Winge, S. B. et al. Transcriptome profiling of fetal Klinefelter testis tissue reveals a possible involvement of long non-coding RNAs in gonocyte maturation. Hum. Mol. Genet. 27, 430–439 (2017).
Cimino, L. et al. Decreased miRNA expression in Klinefelter syndrome. Sci. Rep. 7, 16672 (2017).
Wan, E. S. et al. Genome-wide site-specific differential methylation in the blood of individuals with Klinefelter syndrome. Mol. Reprod. Dev. 82, 377–386 (2015).
Samango-Sprouse, C. et al. Positive effects of early androgen therapy on the behavioral phenotype of boys with 47,XXY. Am. J. Med. Genet. C 169, 150–157 (2015).
Harrington, J. & Palmert, M. R. Distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism: critical appraisal of available diagnostic tests. J. Clin. Endocrinol. Metab. 97, 3056–3067 (2012). This manuscript critically deals with the difficulties in distinguishing CDGP from isolated hypogonadotropic hypogonadism.
Xu, C. et al. Genetic testing facilitates prepubertal diagnosis of congenital hypogonadotropic hypogonadism. Clin. Genet. 92, 213–216 (2017).
Chan, Y.-M., Lippincott, M. F., Kusa, T. O. & Seminara, S. B. Divergent responses to kisspeptin in children with delayed puberty. JCI Insight 3, 99109 (2018).
Stoupa, A. et al. Efficacy and safety of continuous subcutaneous infusion of recombinant human gonadotropins for congenital micropenis during early infancy. Horm. Res. Paediatr. 87, 103–110 (2017).
Main, K., Schmidt, I., Toppari, J. & Skakkebaek, N. Early postnatal treatment of hypogonadotropic hypogonadism with recombinant human FSH and LH. Eur. J. Endocrinol. 146, 75–79 (2002).
Bouvattier, C. et al. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 8, 172–182 (2011).
Ryden, L. et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 34, 3035–3087 (2013).
Corona, G. et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin. Drug Saf. 13, 1327–1351 (2014).
Calof, O. M. et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J. Gerontol. A 60, 1451–1457 (2005).
Haddad, R. M. et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin. Proc. 82, 29–39 (2007).
Fernández-Balsells, M. M. et al. Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 95, 2560–2575 (2010).
Xu, L., Freeman, G., Cowling, B. J. & Schooling, C. M. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 11, 108 (2013).
Borst, S. E. et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 12, 211 (2014).
Albert, S. G. & Morley, J. E. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin. Endocrinol. 85, 436–443 (2016).
Alexander, G. C., Iyer, G., Lucas, E., Lin, D. & Singh, S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Value Health 19, A43 (2016).
Corona, G., Forti, G. & Maggi, M. Why can patients with erectile dysfunction be considered lucky? The association with testosterone deficiency and metabolic syndrome. Aging Male 11, 193–199 (2008).
Thyen, U., Lanz, K., Holterhus, P.-M. & Hiort, O. Epidemiology and initial management of ambiguous genitalia at birth in Germany. Horm. Res. Paediatr. 66, 195–203 (2006).
Klonisch, T., Fowler, P. A. & Hombach-Klonisch, S. Molecular and genetic regulation of testis descent and external genitalia development. Dev. Biol. 270, 1–18 (2004).
Ivell, R. & Anand-Ivell, R. Biological role and clinical significance of insulin-like peptide 3. Curr. Opin. Endocrinol. Diabetes Obes. 18, 210–216 (2011).
Lasala, C., Carré-Eusèbe, D., Picard, J.-Y. & Rey, R. Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 23, 572–585 (2004).
Lamminmäki, A. et al. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm. Behav. 61, 611–616 (2012).
Goldman, A. L. et al. A Reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr. Rev. 38, 302–324 (2017).
Rastrelli, G., Corona, G., Cipriani, S., Mannucci, E. & Maggi, M. Sex hormone-binding globulin is associated with androgen deficiency features independently of total testosterone. Clin. Endocrinol. 88, 556–564 (2018).
Kathrins, M. & Niederberger, C. Diagnosis and treatment of infertility-related male hormonal dysfunction. Nat. Rev. Urol. 13, 309–323 (2016).
Mulhall, J. et al. Evaluation and management of testosterone deficiency: AUA Guideline. AUAnet.org https://www.auanet.org/Documents/Guidelines/PDF/Testosterone%20Website%20Final(0).pdf (2018).
Morales, A. et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Can. Med. Assoc. J. 187, 1369–1377 (2015).
Lunenfeld, B. et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 18, 5–15 (2015).
Author information
Authors and Affiliations
Contributions
Introduction (A.S.); Epidemiology (G.R. and G.H.); Mechanisms/pathophysiology (S.B.S. and I.T.H.); Diagnosis, screening and prevention (R.A.R. and W.J.G.H.); Management (M.R.P., G.C. and G.R.D.); Quality of life (M.K.); Outlook (Y.-M.C. and M.M.); Overview of Primer (A.S.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salonia, A., Rastrelli, G., Hackett, G. et al. Paediatric and adult-onset male hypogonadism. Nat Rev Dis Primers 5, 38 (2019). https://doi.org/10.1038/s41572-019-0087-y
Published:
DOI: https://doi.org/10.1038/s41572-019-0087-y
This article is cited by
-
Insights and implications of sexual dimorphism in osteoporosis
Bone Research (2024)
-
Prevalence and predictors of unrecognised low sexual desire/interest in men with new onset erectile dysfunction: findings from a cross-sectional, real-life study
International Journal of Impotence Research (2024)
-
Stem Leydig cells support macrophage immunological homeostasis through mitochondrial transfer in mice
Nature Communications (2024)
-
The Italian Society of Andrology and Sexual Medicine (SIAMS), along with ten other Italian Scientific Societies, guidelines on the diagnosis and management of erectile dysfunction
Journal of Endocrinological Investigation (2023)
-
Testosterone suppression impacts craniofacial growth structures during puberty
Journal of Orofacial Orthopedics / Fortschritte der Kieferorthopädie (2023)