Abstract
Barrett oesophagus (BE), the only known histological precursor of oesophageal adenocarcinoma (EAC), is a condition in which the squamous epithelium of the oesophagus is replaced by columnar epithelium as an adaptive response to gastro-oesophageal reflux. EAC has one of the fastest rising incidences of cancers in Western countries and has a dismal prognosis. BE is usually detected during endoscopic examination, and diagnosis is confirmed by the histological presence of intestinal metaplasia. Advances in genomics and transcriptomics have improved our understanding of the pathogenesis and malignant progression of intestinal metaplasia. As the majority of EAC cases are diagnosed in individuals without a known history of BE, screening for BE could potentially decrease disease-related mortality. Owing to the pre-malignant nature of BE, endoscopic surveillance of patients with BE is imperative for early detection and treatment of dysplasia to prevent further progression to invasive EAC. Developments in endoscopic therapy have resulted in a major shift in the treatment of patients with BE who have dysplasia or early EAC, from surgical resection to endoscopic resection and ablation. In addition to symptom control by optimization of lifestyle and pharmacological therapy with proton pump inhibitors, chemopreventive strategies based on NSAIDs and statins are currently being investigated for BE management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Spechler, S. J., Sharma, P., Souza, R. F., Inadomi, J. M. & Shaheen, N. J. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 140, 1084–1091 (2011).
Shaheen, N. J., Falk, G. W., Iyer, P. G. & Gerson, L. B. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 111, 30–50 (2016).
Fitzgerald, R. C. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 63, 7–42 (2014).
Evans, J. A. et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest. Endosc. 76, 1087–1094 (2012).
Ronkainen, J. et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 129, 1825–1831 (2005).
Rex, D. K. et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 125, 1670–1677 (2003).
Sawas, T. et al. Identification of prognostic phenotypes of esophageal adenocarcinoma in two independent cohorts. Gastroenterology 155, 1720–1728 (2018).
Ross-Innes, C. S. et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLOS Med. 12, e1001780 (2015).
Vaughan, T. L. & Fitzgerald, R. C. Precision prevention of oesophageal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 12, 243–248 (2015).
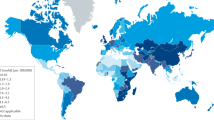
Arnold, M., Laversanne, M., Brown, L. M., Devesa, S. S. & Bray, F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am. J. Gastroenterol. 112, 1247–1255 (2017).
Jung, K. W. et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am. J. Gastroenterol. 106, 1447–1455 (2011).
Alcedo, J. et al. Trends in Barrett’s esophagus diagnosis in Southern Europe: implications for surveillance. Dis. Esophagus 22, 239–248 (2009).
Kadri, S. R. et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 341, c4372 (2010). This prospective study screens individuals with long-term PPI use for BE using the minimally invasive Cytosponge.
Lagergren, J., Bergstrom, R., Lindgren, A. & Nyren, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 340, 825–831 (1999).
Lin, E. C., Holub, J., Lieberman, D. & Hur, C. Low prevalence of suspected Barrett’s esophagus in patients with gastroesophageal reflux disease without alarm symptoms. Clin. Gastroenterol. Hepatol. 17, 857–863 (2018).
Shaheen, N. J., Crosby, M. A., Bozymski, E. M. & Sandler, R. S. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology 119, 333–338 (2000).
Hvid-Jensen, F., Pedersen, L., Drewes, A. M., Sorensen, H. T. & Funch-Jensen, P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 365, 1375–1383 (2011).
Peters, Y. et al. Incidence of progression of persistent non-dysplastic Barrett’s esophagus to malignancy. Clin. Gastroenterol. Hepatol. 17, 869–877 (2018).
Olsen, C. M. et al. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am. J. Epidemiol. 174, 582–590 (2011).
Abrams, J. A., Fields, S., Lightdale, C. J. & Neugut, A. I. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin. Gastroenterol. Hepatol. 6, 30–34 (2008).
Wang, A., Mattek, N. C., Holub, J. L., Lieberman, D. A. & Eisen, G. M. Prevalence of complicated gastroesophageal reflux disease and Barrett’s esophagus among racial groups in a multi-center consortium. Dig. Dis. Sci. 54, 964–971 (2009).
Keyashian, K. et al. Barrett’s esophagus in Latinos undergoing endoscopy for gastroesophageal reflux disease symptoms. Dis. Esophagus 26, 44–49 (2013).
Shiota, S., Singh, S., Anshasi, A. & El-Serag, H. B. Prevalence of Barrett’s esophagus in Asian countries: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 13, 1907–1918 (2015).
Cook, M. B., Wild, C. P. & Forman, D. A systematic review and meta-analysis of the sex ratio for Barrett’s esophagus, erosive reflux disease, and nonerosive reflux disease. Am. J. Epidemiol. 162, 1050–1061 (2005).
Edelstein, Z. R., Bronner, M. P., Rosen, S. N. & Vaughan, T. L. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am. J. Gastroenterol. 104, 834–842 (2009).
Westra, W. M. et al. Smokeless tobacco and cigar and/or pipe are risk factors for Barrett esophagus in male patients with gastroesophageal reflux disease. Mayo Clin. Proc. 93, 1282–1289 (2018).
Cook, M. B. et al. Cigarette smoking increases risk of Barrett’s esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Gastroenterology 142, 744–753 (2012).
Zhao, Z. et al. Dietary fruit, vegetable, fat, and red and processed meat intakes and Barrett’s esophagus risk: a systematic review and meta-analysis. Sci. Rep. 6, 27334 (2016).
Keszei, A. P. et al. Meat consumption and the risk of Barrett’s esophagus in a large Dutch cohort. Cancer Epidemiol. Biomarkers Prev. 22, 1162–1166 (2013).
Iijima, K. et al. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology 122, 1248–1257 (2002).
Moriya, A. et al. In vitro studies indicate that acid catalysed generation of N-nitrosocompounds from dietary nitrate will be maximal at the gastro-oesophageal junction and cardia. Scand. J. Gastroenterol. 37, 253–261 (2002).
Thrift, A. P. et al. Lifetime alcohol consumption and risk of Barrett’s esophagus. Am. J. Gastroenterol. 106, 1220–1230 (2011).
Kubo, A. et al. Alcohol types and sociodemographic characteristics as risk factors for Barrett’s esophagus. Gastroenterology 136, 806–815 (2009).
Singh, S. et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 11, 1399–1412 (2013).
Kubo, A. et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the International BEACON Consortium. Gut 62, 1684–1691 (2013).
Chandar, A. K. et al. Association of serum levels of adipokines and insulin with risk of Barrett’s esophagus: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 13, 2241–2255 (2015).
Ayazi, S. et al. Obesity and gastroesophageal reflux: quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J. Gastrointest. Surg. 13, 1440–1447 (2009).
Wu, J. C., Mui, L. M., Cheung, C. M., Chan, Y. & Sung, J. J. Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 132, 883–889 (2007).
Leodolter, A. et al. Progression of specialized intestinal metaplasia at the cardia to macroscopically evident Barrett’s esophagus: an entity of concern in the ProGERD study. Scand. J. Gastroenterol. 47, 1429–1435 (2012).
Dunbar, K. B. et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA 315, 2104–2112 (2016).
Iascone, C., DeMeester, T. R., Little, A. G. & Skinner, D. B. Barrett’s esophagus. Functional assessment, proposed pathogenesis, and surgical therapy. Arch. Surg. 118, 543–549 (1983).
Collen, M. J., Lewis, J. H. & Benjamin, S. B. Gastric acid hypersecretion in refractory gastroesophageal reflux disease. Gastroenterology 98, 654–661 (1990).
Mulholland, M. W., Reid, B. J., Levine, D. S. & Rubin, C. E. Elevated gastric acid secretion in patients with Barrett’s metaplastic epithelium. Dig. Dis. Sci. 34, 1329–1334 (1989).
Taylor, J. B. & Rubenstein, J. H. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. Am. J. Gastroenterol. 105, 1730–1737 (2010).
Ward, E. M. et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am. J. Gastroenterol. 101, 12–17 (2006).
Andrici, J., Tio, M., Cox, M. R. & Eslick, G. D. Hiatal hernia and the risk of Barrett’s esophagus. J. Gastroenterol. Hepatol. 28, 415–431 (2013).
Wang, Z. et al. Helicobacter pylori infection is associated with reduced risk of Barrett’s esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Am. J. Gastroenterol. 113, 1148–1155 (2018).
Eross, B. et al. Helicobacter pylori infection reduces the risk of Barrett’s esophagus: a meta-analysis and systematic review. Helicobacter 23, e12504 (2018).
Chak, A. et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol. Biomarkers Prev. 15, 1668–1673 (2006).
Verbeek, R. E. et al. Familial clustering of Barrett’s esophagus and esophageal adenocarcinoma in a European cohort. Clin. Gastroenterol. Hepatol. 12, 1656–1663 (2014).
Thrift, A. P. et al. The use of nonsteroidal anti-inflammatory drugs and the risk of Barrett’s oesophagus. Aliment. Pharmacol. Ther. 34, 1235–1244 (2011).
Beales, I. L., Dearman, L., Vardi, I. & Loke, Y. Reduced risk of Barrett’s esophagus in statin users: case-control study and meta-analysis. Dig. Dis. Sci. 61, 238–246 (2016).
Brown, C. S. et al. Predicting progression in Barrett’s esophagus: development and validation of the Barrett’s Esophagus Assessment of Risk Score (BEAR Score). Ann. Surg. 267, 716–720 (2018).
Gatenby, P., Bhattacharjee, S., Wall, C., Caygill, C. & Watson, A. Risk stratification for malignant progression in Barrett’s esophagus: gender, age, duration and year of surveillance. World J. Gastroenterol. 22, 10592–10600 (2016).
Krishnamoorthi, R. et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 16, 1046–1055 (2018).
Parasa, S. et al. Development and validation of a model to determine risk of progression of Barrett’s esophagus to neoplasia. Gastroenterology 154, 1282–1289 (2018). This study of 2,697 patients with BE develops a scoring system based on male sex, smoking, length of BE and baseline LGD and identifies patients with BE at low risk, intermediate risk and high risk of EAC.
Bhat, S. et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J. Natl Cancer Inst. 103, 1049–1057 (2011).
Kelty, C. J., Gough, M. D., Van Wyk, Q., Stephenson, T. J. & Ackroyd, R. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand. J. Gastroenterol. 42, 1271–1274 (2007).
Codipilly, D. C. et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 154, 2068–2086 (2018). This systematic review and meta-analysis shows that surveillance of patients with BE is associated with detection of earlier-stage EAC and a small survival benefit.
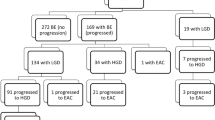
Duits, L. C. et al. Patients with Barrett’s esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to neoplasia. Gastroenterology 152, 993–1001 (2017).
Kestens, C., Offerhaus, G. J., van Baal, J. W. & Siersema, P. D. Patients with Barrett’s esophagus and persistent low-grade dysplasia have an increased risk for high-grade dysplasia and cancer. Clin. Gastroenterol. Hepatol. 14, 956–962 (2016).
Rastogi, A. et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest. Endosc. 67, 394–398 (2008).
Shaheen, N. J. et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. Engl. J. Med. 360, 2277–2288 (2009). This sham-controlled randomized trial analyses whether endoscopic RFA could eradicate dysplastic BE and decrease the rate of malignant progression.
Overholt, B. F. et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest. Endosc. 62, 488–498 (2005).
Souza, R. F. Reflux esophagitis and its role in the pathogenesis of Barrett’s metaplasia. J. Gastroenterol. 52, 767–776 (2017).
Glickman, J. N. et al. Mucin core polypeptide expression in the progression of neoplasia in Barrett’s esophagus. Hum. Pathol. 37, 1304–1315 (2006).
Lavery, D. L. et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut 63, 1854–1863 (2014).
McQuaid, K. R., Laine, L., Fennerty, M. B., Souza, R. & Spechler, S. J. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment. Pharmacol. Ther. 34, 146–165 (2011).
Mari, L. et al. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep. 7, 1197–1210 (2014).
Wang, D. H. et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology 138, 1810–1822 (2010).
Jiang, M. et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550, 529–533 (2017).
Kong, J., Crissey, M. A., Funakoshi, S., Kreindler, J. L. & Lynch, J. P. Ectopic Cdx2 expression in murine esophagus models an intermediate stage in the emergence of Barrett’s esophagus. PLOS ONE 6, e18280 (2011).
Vega, M. E. et al. Inhibition of Notch signaling enhances transdifferentiation of the esophageal squamous epithelium towards a Barrett’s-like metaplasia via KLF4. Cell Cycle 13, 3857–3866 (2014).
Slack, J. M. Metaplasia and transdifferentiation: from pure biology to the clinic. Nature reviews. Mol. Cell Biol. 8, 369–378 (2007).
Groisman, G. M., Amar, M. & Meir, A. Expression of the intestinal marker Cdx2 in the columnar-lined esophagus with and without intestinal (Barrett’s) metaplasia. Mod. Pathol. 17, 1282–1288 (2004).
Barbera, M. & Fitzgerald, R. C. Cellular origin of Barrett’s metaplasia and oesophageal stem cells. Biochem. Soc. Trans. 38, 370–373 (2010).
Li, H. et al. Mechanisms of columnar metaplasia and squamous regeneration in experimental Barrett’s esophagus. Surgery 115, 176–181 (1994).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012).
Kübler, K. et al. Tumor mutational landscape is a record of the pre-malignant state. Preprint at bioRxiv https://doi.org/10.1101/517565 (2019).
The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017).
Coad, R. A. et al. On the histogenesis of Barrett’s oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J. Pathol. 206, 388–394 (2005).
Leedham, S. J. et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut 57, 1041–1048 (2008).
Kruger, L. et al. Ductular and proliferative response of esophageal submucosal glands in a porcine model of esophageal injury and repair. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G180–G191 (2017).
von Furstenberg, R. J. et al. Porcine esophageal submucosal gland culture model shows capacity for proliferation and differentiation. Cell. Mol. Gastroenterol. Hepatol. 4, 385–404 (2017).
Owen, R. P. et al. Single cell RNA-seq reveals profound transcriptional similarity between Barrett’s oesophagus and oesophageal submucosal glands. Nat. Commun. 9, 4261 (2018).
Wang, X. et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 145, 1023–1035 (2011).
Seery, J. P. Stem cells of the oesophageal epithelium. J. Cell Sci. 115, 1783–1789 (2002).
Hutchinson, L. et al. Human Barrett’s adenocarcinoma of the esophagus, associated myofibroblasts, and endothelium can arise from bone marrow-derived cells after allogeneic stem cell transplant. Stem Cells Dev. 20, 11–17 (2011).
Kapoor, H., Lohani, K. R., Lee, T. H., Agrawal, D. K. & Mittal, S. K. Animal models of Barrett’s esophagus and esophageal adenocarcinoma-past, present, and future. Clin. Transl Sci. 8, 841–847 (2015).
Dong, J. et al. Interactions between genetic variants and environmental factors affect risk of esophageal adenocarcinoma and Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 16, 1598–1606 (2018).
Dong, J. et al. Determining risk of Barrett’s esophagus and esophageal adenocarcinoma based on epidemiologic factors and genetic variants. Gastroenterology 154, 1273–1281 (2018).
Sun, X. et al. Genomic regions associated with susceptibility to Barrett’s esophagus and esophageal adenocarcinoma in African Americans: the cross BETRNet admixture study. PLOS ONE 12, e0184962 (2017).
Ek, W. E. et al. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett’s esophagus, and gastroesophageal reflux. J. Natl Cancer Inst. 105, 1711–1718 (2013).
Gharahkhani, P. et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett’s oesophagus: a large-scale meta-analysis. Lancet. Oncol. 17, 1363–1373 (2016). This meta-analysis of all GWASs of BE and EAC investigates novel genetic risk variants for BE development and malignant progression.
Buas, M. F. et al. Germline variation in inflammation-related pathways and risk of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut 66, 1739–1747 (2017).
Dai, J. Y. et al. A newly identified susceptibility locus near FOXP1 modifies the association of gastroesophageal reflux with Barrett’s esophagus. Cancer Epidemiol. Biomarkers Prev. 24, 1739–1747 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Sikkema, M. et al. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett’s esophagus: a case-control study. Am. J. Gastroenterol. 104, 2673–2680 (2009).
Souza, R. F., Morales, C. P. & Spechler, S. J. Review article: a conceptual approach to understanding the molecular mechanisms of cancer development in Barrett’s oesophagus. Aliment. Pharmacol. Ther. 15, 1087–1100 (2001).
Stachler, M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 47, 1047–1055 (2015). This paper investigates the malignant progression of BE to EAC by whole-exome sequencing and shows that the emergence of EAC follows a more rapid pathway that involves genome duplication.
Ross-Innes, C. S. et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat. Genet. 47, 1038–1046 (2015).
Weaver, J. M. J. et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat. Genet. 46, 837–843 (2014).
Frankell, A. M. et al. The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat. Genet. 51, 506–516 (2019).
Martinez, P. et al. Dynamic clonal equilibrium and predetermined cancer risk in Barrett’s oesophagus. Nat. Commun. 7, 12158 (2016).
Newell, F. et al. Complex structural rearrangements are present in high-grade dysplastic Barrett’s oesophagus samples. BMC Med. Genomics 12, 31 (2019).
Nones, K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 5, 5224 (2014).
Moayyedi, P. M. et al. ACG and CAG clinical guideline: management of dyspepsia. Am. J. Gastroenterol. 112, 988–1013 (2017).
Muthusamy, V. R. et al. The role of endoscopy in the management of GERD. Gastrointest. Endosc. 81, 1305–1310 (2015).
Weusten, B. et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 49, 191–198 (2017).
Iwakiri, K. et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J. Gastroenterol. 51, 751–767 (2016).
Whiteman, D. C. et al. Australian clinical practice guidelines for the diagnosis and management of Barrett’s esophagus and early esophageal adenocarcinoma. J. Gastroenterol. Hepatol. 30, 804–820 (2015).
Pohl, H. et al. Length of Barrett’s oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut 65, 196–201 (2016).
Thota, P. N. et al. Low risk of high-grade dysplasia or esophageal adenocarcinoma among patients with Barrett’s esophagus less than 1cm (irregular Z line) within 5 years of index endoscopy. Gastroenterology 152, 987–992 (2017).
Sharma, P. et al. Quality indicators for the management of Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma: international consensus recommendations from the American Gastroenterological Association Symposium. Gastroenterology 149, 1599–1606 (2015).
Sharma, P. et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 131, 1392–1399 (2006).
Antony, A. et al. Adherence to quality indicators in endoscopic surveillance of Barrett’s esophagus and correlation to dysplasia detection rates. Clin. Res. Hepatol. Gastroenterol. 42, 591–596 (2018).
Ooi, J. et al. Dedicated Barrett’s surveillance sessions managed by trained endoscopists improve dysplasia detection rate. Endoscopy 49, C1 (2017).
Harrison, R. et al. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am. J. Gastroenterol. 102, 1154–1161 (2007).
Hirota, W. K. et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology 116, 277–285 (1999).
Schlemper, R. J. et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47, 251–255 (2000).
Vennalaganti, P. et al. Discordance among pathologists in the United States and Europe in diagnosis of low-grade dysplasia for patients with Barrett’s esophagus. Gastroenterology 152, 564–570 (2017).
Abela, J. E. et al. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am. J. Gastroenterol. 103, 850–855 (2008).
Vennalaganti, P. R. et al. Increased detection of Barrett’s esophagus-associated neoplasia using wide-area trans-epithelial sampling: a multicenter, prospective, randomized trial. Gastrointest. Endosc. 87, 348–355 (2018).
Sharma, P. et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut 62, 15–21 (2013).
Waterhouse, D. J., Fitzpatrick, C. R. M., di Pietro, M. & Bohndiek, S. E. Emerging optical methods for endoscopic surveillance of Barrett’s oesophagus. Lancet Gastroenterol. Hepatol. 3, 349–362 (2018).
Visrodia, K. et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: a systematic review and meta-analysis. Gastroenterology 150, 599–607 (2016).
World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective, Continuous Update Project Expert Report (World Cancer Research Fund International, 2018).
Lam, S., Alexandre, L., Luben, R. & Hart, A. R. The association between physical activity and the risk of symptomatic Barrett’s oesophagus: a UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 30, 71–75 (2018).
Kunzmann, A. T. et al. Physical activity, sedentary behaviour and risk of oesophago-gastric cancer: a prospective cohort study within UK Biobank. United European Gastroenterol. J. 6, 1144–1154 (2018).
Realdon, S. et al. Adherence to WCRF/AICR lifestyle recommendations for cancer prevention and the risk of Barrett’s esophagus onset and evolution to esophageal adenocarcinoma: results from a pilot study in a high-risk population. Eur. J. Nutr. 55, 1563–1571 (2016).
Mulholland, H. G., Murray, L. J., Anderson, L. A., Cantwell, M. M. & FINBAR study group. Vitamin D, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br. J. Nutr. 106, 732–741 (2011).
Dai, Q. et al. Dietary magnesium, calcium:magnesium ratio and risk of reflux oesophagitis, Barrett’s oesophagus and oesophageal adenocarcinoma: a population-based case-control study. Br. J. Nutr. 115, 342–350 (2016).
Garland, C. F. et al. The role of vitamin D in cancer prevention. Am. J. Public Health 96, 252–261 (2006).
Dibaba, D. T., Xun, P. & He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review. Eur. J. Clin. Nutr. 68, 510–516 (2014).
Vaezi, M. F., Yang, Y. X. & Howden, C. W. Complications of proton pump inhibitor therapy. Gastroenterology 153, 35–48 (2017).
Attwood, S. E. et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment. Pharmacol. Ther. 41, 1162–1174 (2015).
O’Sullivan, K. E. et al. The role of inflammation in cancer of the esophagus. Expert Rev. Gastroenterol. Hepatol. 8, 749–760 (2014).
Ouatu-Lascar, R., Fitzgerald, R. C. & Triadafilopoulos, G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology 117, 327–335 (1999).
Abu-Sneineh, A. et al. The effects of high-dose esomeprazole on gastric and oesophageal acid exposure and molecular markers in Barrett’s oesophagus. Aliment. Pharmacol. Ther. 32, 1023–1030 (2010).
Singh, S., Garg, S. K., Singh, P. P., Iyer, P. G. & El-Serag, H. B. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut 63, 1229–1237 (2014).
Jankowski, J. A. Z. et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet 392, 400–408 (2018). This randomized trial of 2,557 patients with BE shows that the combination of high-dose PPI and aspirin is the best strategy to reduce the risk of EAC and HGD.
Liao, L. M. et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 142, 442–452 (2012).
Zhang, S. et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta-analysis. Br. J. Cancer 110, 2378–2388 (2014).
Ogunwobi, O. O. & Beales, I. L. Statins inhibit proliferation and induce apoptosis in Barrett’s esophageal adenocarcinoma cells. Am. J. Gastroenterol. 103, 825–837 (2008).
Nguyen, T., Khalaf, N., Ramsey, D. & El-Serag, H. B. Statin use is associated with a decreased risk of Barrett’s esophagus. Gastroenterology 147, 314–323 (2014).
Nguyen, T., Duan, Z., Naik, A. D., Kramer, J. R. & El-Serag, H. B. Statin use reduces risk of esophageal adenocarcinoma in US veterans with Barrett’s esophagus: a nested case-control study. Gastroenterology 149, 1392–1398 (2015).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013).
Verbeek, R. E. et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am. J. Gastroenterol. 109, 1215–1222 (2014).
Sampliner, R. E. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am. J. Gastroenterol. 93, 1028–1032 (1998).
Stoltey, J., Reeba, H., Ullah, N., Sabhaie, P. & Gerson, L. Does Barrett’s oesophagus develop over time in patients with chronic gastro-oesophageal reflux disease? Aliment. Pharmacol. Ther. 25, 83–91 (2007).
Rubenstein, J. H. et al. Prediction of Barrett’s esophagus among men. Am. J. Gastroenterol. 108, 353–362 (2013). This article presents a study in which a prediction model for BE based on GERD, age, abdominal obesity and cigarette use with an AUC of 0.72 is developed in 822 male patients with colorectal cancer who are screened using upper endoscopy.
Thrift, A. P., Vaughan, T. L., Anderson, L. A., Whiteman, D. C. & El-Serag, H. B. External validation of the Michigan Barrett’s esophagus prediction tool. Clin. Gastroenterol. Hepatol. 15, 1124–1126 (2017).
Thrift, A. P., Garcia, J. M. & El-Serag, H. B. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 12, 1267–1271 (2014).
Spechler, S. J., Katzka, D. A. & Fitzgerald, R. C. New screening techniques in Barrett’s esophagus: great ideas or great practice? Gastroenterology 154, 1594–1601 (2018).
Sami, S. S. & Iyer, P. G. Recent advances in screening for Barrett’s esophagus. Curr. Treat. Options Gastroenterol. 16, 1–14 (2018).
Sami, S. S. et al. Performance characteristics of unsedated ultrathin video endoscopy in the assessment of the upper GI tract: systematic review and meta-analysis. Gastrointest. Endosc. 82, 782–792 (2015).
Sami, S. S. et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett’s esophagus screening in the community. Am. J. Gastroenterol. 110, 148–158 (2015). This randomized controlled trial shows that mobile and outpatient unsedated TNE may be an effective alternative to standard endoscopy to screen for BE.
Moriarty, J. P. et al. Costs associated with Barrett’s esophagus screening in the community: an economic analysis of a prospective randomized controlled trial of sedated versus hospital unsedated versus mobile community unsedated endoscopy. Gastrointest. Endosc. 87, 88–94 (2018).
Sami, S. S. et al. Acceptability, accuracy, and safety of disposable transnasal capsule endoscopy for Barrett’s esophagus screening. Clin. Gastroenterol. Hepatol. 17, 638–646 (2018).
Alashkar, B. et al. Development of a program to train physician extenders to perform transnasal esophagoscopy and screen for Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 12, 785–792 (2014).
Sharma, P. et al. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett’s esophagus: a blinded, prospective study. Am. J. Gastroenterol. 103, 525–532 (2008).
Bhardwaj, A., Hollenbeak, C. S., Pooran, N. & Mathew, A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am. J. Gastroenterol. 104, 1533–1539 (2009).
Offman, J. et al. Barrett’s oESophagus trial 3 (BEST3): study protocol for a randomised controlled trial comparing the Cytosponge-TFF3 test with usual care to facilitate the diagnosis of oesophageal pre-cancer in primary care patients with chronic acid reflux. BMC Cancer 18, 784 (2018).
Iyer, P. G. et al. Highly discriminant methylated DNA markers for the non-endoscopic detection of Barrett’s esophagus. Am. J. Gastroenterol. 113, 1156–1166 (2018).
Moinova, H. R. et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl Med. 10, eaao5848 (2018).
Chettouh, H. et al. Methylation panel is a diagnostic biomarker for Barrett’s oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut 67, 1942–1949 (2018).
Li, X. et al. Selection and application of tissue microRNAs for nonendoscopic diagnosis of Barrett’s esophagus. Gastroenterology 155, 771–783 (2018).
Chan, D. K. et al. Breath testing for Barrett’s esophagus using exhaled volatile organic compound profiling with an electronic nose device. Gastroenterology 152, 24–26 (2017).
Honing, J. et al. Endosheath ultrathin transnasal endoscopy is a cost-effective method for screening for Barrett’s esophagus in patients with GERD symptoms. Gastrointest. Endosc. 89, 712–722 (2018).
Nietert, P. J. et al. Cost-effectiveness of screening a population with chronic gastroesophageal reflux. Gastrointest. Endosc. 57, 311–318 (2003).
Inadomi, J. M. et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann. Intern. Med. 138, 176–186 (2003).
Gerson, L. B., Groeneveld, P. W. & Triadafilopoulos, G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin. Gastroenterol. Hepatol. 2, 868–879 (2004).
Heberle, C. R. et al. Cost effectiveness of screening patients with gastroesophageal reflux disease for Barrett’s esophagus with a minimally invasive cell sampling device. Clin. Gastroenterol. Hepatol. 15, 1397–1404 (2017).
Wani, S. et al. Endoscopic eradication therapy for patients with Barrett’s esophagus-associated dysplasia and intramucosal cancer. Gastrointest. Endosc. 87, 907–931 (2018).
Corley, D. A. et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology 145, 312–319 (2013).
Holmberg, D., Ness-Jensen, E., Mattsson, F. & Lagergren, J. Clinical prediction model for tumor progression in Barrett’s esophagus. Surg. Endosc. https://doi.org/10.1007/s00464-018-6590-5 (2018).
Peters, F. P. et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest. Endosc. 67, 604–609 (2008).
Markar, S. R. et al. The influence of procedural volume and proficiency gain on mortality from upper GI endoscopic mucosal resection. Gut 67, 79–85 (2018).
Pech, O. et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 146, 652–660 (2014).
Subramaniam, S. et al. Complex early Barrett’s neoplasia at 3 Western centers: European Barrett’s Endoscopic Submucosal Dissection Trial (E-BEST). Gastrointest. Endosc. 86, 608–618 (2017).
Pimentel-Nunes, P. et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 47, 829–854 (2015).
Terheggen, G. et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 66, 783–793 (2017).
Yang, D. et al. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest. Endosc. 87, 1383–1393 (2018).
Tomizawa, Y. et al. Safety of endoscopic mucosal resection for Barrett’s esophagus. Am. J. Gastroenterol. 108, 1440–1447 (2013).
Shaheen, N. J. et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 141, 460–468 (2011).
Phoa, K. N. et al. Radiofrequency ablation versus endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 311, 1209–1217 (2014).
Manner, H. et al. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation versus surveillance (APE study). Endoscopy 46, 6–12 (2014).
Thota, P. N. et al. Cryotherapy and radiofrequency ablation for eradication of Barrett’s esophagus with dysplasia or intramucosal cancer. Dig. Dis. Sci. 63, 1311–1319 (2018).
Dumot, J. A. et al. An open-label, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest. Endosc. 70, 635–644 (2009).
Milashka, M. et al. Sixteen-year follow-up of Barrett’s esophagus, endoscopically treated with argon plasma coagulation. United European Gastroenterol. J. 2, 367–373 (2014).
Manner, H. et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (Hybrid-APC) versus standard APC: a randomized ex-vivo study. United European Gastroenterol. J. 2, 383–390 (2014).
Orman, E. S., Li, N. & Shaheen, N. J. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 11, 1245–1255 (2013).
Anders, M. et al. Subsquamous extension of intestinal metaplasia is detected in 98% of cases of neoplastic Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 12, 405–410 (2014).
Gray, N. A., Odze, R. D. & Spechler, S. J. Buried metaplasia after endoscopic ablation of Barrett’s esophagus: a systematic review. Am. J. Gastroenterol. 106, 1899–1908 (2011).
Belghazi, K. et al. Long-term follow-up results of stepwise radical endoscopic resection for Barrett’s esophagus with early neoplasia. Gastrointest. Endosc. 87, 77–84 (2018).
Desai, M. et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest. Endosc. 85, 482–495 (2017).
Cho, J. W. et al. Lymph node metastases in esophageal carcinoma: an endoscopist’s view. Clin. Endosc. 47, 523–529 (2014).
Manner, H. et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am. J. Gastroenterol. 103, 2589–2597 (2008).
Manner, H. et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin. Gastroenterol. Hepatol. 11, 630–635 (2013).
Cotton, C. C., Haidry, R., Thrift, A. P., Lovat, L. & Shaheen, N. J. Development of evidence-based surveillance intervals after radiofrequency ablation of Barrett’s esophagus. Gastroenterology 155, 316–326 (2018). This article provides evidence-based surveillance intervals for patients with endoscopically treated BE.
Britton, J. et al. Effect of diagnosis, surveillance, and treatment of Barrett’s oesophagus on health-related quality of life. Lancet Gastroenterol. Hepatol. 3, 57–65 (2018). This paper presents a comprehensive review of the QOL in patients with BE.
Kamolz, T., Pointner, R. & Velanovich, V. The impact of gastroesophageal reflux disease on quality of life. Surg. Endosc. 17, 1193–1199 (2003).
Ofman, J. J. The economic and quality-of-life impact of symptomatic gastroesophageal reflux disease. Am. J. Gastroenterol. 98, S8–S14 (2003).
Eloubeidi, M. A. & Provenzale, D. Health-related quality of life and severity of symptoms in patients with Barrett’s esophagus and gastroesophageal reflux disease patients without Barrett’s esophagus. Am. J. Gastroenterol. 95, 1881–1887 (2000).
Kulig, M. et al. Quality of life in relation to symptoms in patients with gastro-oesophageal reflux disease — an analysis based on the ProGERD initiative. Aliment. Pharmacol. Ther. 18, 767–776 (2003).
Lippmann, Q. K., Crockett, S. D., Dellon, E. S. & Shaheen, N. J. Quality of life in GERD and Barrett’s esophagus is related to gender and manifestation of disease. Am. J. Gastroenterol. 104, 2695–2703 (2009).
Rodger, A. J., Jolley, D., Thompson, S. C., Lanigan, A. & Crofts, N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology 30, 1299–1301 (1999).
Rubenstein, J. H. & Inadomi, J. M. Defining a clinically significant adverse impact of diagnosing Barrett’s esophagus. J. Clin. Gastroenterol. 40, 109–115 (2006).
Essink-Bot, M. L. et al. Different perceptions of the burden of upper GI endoscopy: an empirical study in three patient groups. Qual. Life Res. 16, 1309–1318 (2007).
Cooper, S. C. et al. Endoscopic surveillance for Barrett’s oesophagus: the patients’ perspective. Eur. J. Gastroenterol. Hepatol. 21, 850–854 (2009).
Levine, D. S., Blount, P. L., Rudolph, R. E. & Reid, B. J. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am. J. Gastroenterol. 95, 1152–1157 (2000).
Kruijshaar, M. E. et al. The burden of upper gastrointestinal endoscopy in patients with Barrett’s esophagus. Endoscopy 38, 873–878 (2006).
Gerson, L. B., Ullah, N., Hastie, T. & Goldstein, M. K. Does cancer risk affect health-related quality of life in patients with Barrett’s esophagus? Gastrointest. Endosc. 65, 16–25 (2007).
Macrae, F. A., Hill, D. J., St John, D. J., Ambikapathy, A. & Garner, J. F. Predicting colon cancer screening behavior from health beliefs. Prev. Med. 13, 115–126 (1984).
Kruijshaar, M. E. et al. Patients with Barrett’s esophagus perceive their risk of developing esophageal adenocarcinoma as low. Gastrointest. Endosc. 65, 26–30 (2007).
van der Ende-van Loon, M. C. M., Rosmolen, W. D., Houterman, S., Schoon, E. J. & Curvers, W. L. Cancer risk perception in relation to associated symptoms in Barrett’s patients: a cross sectional study on quality of life. United European Gastroenterol. J. 6, 1316–1322 (2018).
Shaheen, N. J. et al. The perception of cancer risk in patients with prevalent Barrett’s esophagus enrolled in an endoscopic surveillance program. Gastroenterology 129, 429–436 (2005).
Stier, M. W. et al. Perceptions of risk and therapy among patients with Barrett’s esophagus: a patient survey study. Dis. Esophagus 31, dox109 (2018).
Smyth, E. C. et al. Oesophageal cancer. Nat. Rev. Dis. Primers 3, 17048 (2017).
Shaheen, N. J. et al. Quality of life following radiofrequency ablation of dysplastic Barrett’s esophagus. Endoscopy 42, 790–799 (2010).
Rosmolen, W. D. et al. Quality of life and fear of cancer recurrence after endoscopic treatment for early Barrett’s neoplasia: a prospective study. Dis. Esophagus 30, 1–9 (2017).
Rosmolen, W. D. et al. Quality of life and fear of cancer recurrence after endoscopic and surgical treatment for early neoplasia in Barrett’s esophagus. Endoscopy 42, 525–531 (2010).
Haidry, R. J. et al. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett’s oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut 64, 1192–1199 (2015).
Nguyen, T., Thrift, A. P., Yu, X., Duan, Z. & El-Serag, H. B. The annual risk of esophageal adenocarcinoma does not decrease over time in patients with Barrett’s esophagus. Am. J. Gastroenterol. 112, 1049–1055 (2017).
Old, O. et al. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J. Med. Screen. 22, 158–164 (2015). This article presents a study protocol for a randomized study that will provide data to evaluate the efficacy and cost-effectiveness of screening patients with BE for the presence of EAC.
Visrodia, K. et al. Cryotherapy for persistent Barrett’s esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest. Endosc. 87, 1396–1404 (2018).
Kazumori, H., Ishihara, S., Takahashi, Y., Amano, Y. & Kinoshita, Y. Roles of Kruppel-like factor 4 in oesophageal epithelial cells in Barrett’s epithelium development. Gut 60, 608–617 (2011).
Wang, D. H. et al. Hedgehog signaling regulates FOXA2 in esophageal embryogenesis and Barrett’s metaplasia. J. Clin. Invest. 124, 3767–3780 (2014).
Andrici, J., Cox, M. R. & Eslick, G. D. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 28, 1258–1273 (2013).
Anderson, L. A. et al. The association between alcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology 136, 799–805 (2009).
Rokkas, T., Pistiolas, D., Sechopoulos, P., Robotis, I. & Margantinis, G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin. Gastroenterol. Hepatol. 5, 1413–1417 (2007).
Salomao, M. A., Lam-Himlin, D. & Pai, R. K. Substantial interobserver agreement in the diagnosis of dysplasia in Barrett esophagus upon review of a patient’s entire set of biopsies. Am. J. Surg. Pathol. 42, 376–381 (2018).
Montgomery, E. et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum. Pathol. 32, 368–378 (2001).
Desai, T. K. et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut 61, 970–976 (2012).
Sonwalkar, S. A. et al. A study of indefinite for dysplasia in Barrett’s oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (alpha-methylacyl-CoA-racemase). Histopathology 56, 900–907 (2010).
Kestens, C., Leenders, M., Offerhaus, G. J., van Baal, J. W. & Siersema, P. D. Risk of neoplastic progression in Barrett’s esophagus diagnosed as indefinite for dysplasia: a nationwide cohort study. Endoscopy 47, 409–414 (2015).
Singh, S. et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest. Endosc. 79, 897–909 (2014).
Grotenhuis, B. A. et al. Inter- and intraobserver variation in the histopathological evaluation of early oesophageal adenocarcinoma. J. Clin. Pathol. 63, 978–981 (2010).
Kerkhof, M. et al. Grading of dysplasia in Barrett’s oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology 50, 920–927 (2007).
Acknowledgements
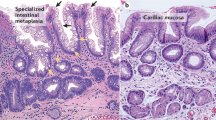
The authors thank R. S. van der Post and M. O’Donovan for providing the histopathological images.
Reviewer information
Nature Reviews Disease Primers thanks S. McDonald, A. Bansal, M. Jansen, R. Odze, K. Krishnadath, H. Barr and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Introduction (Y.P. and A.A.-K.); Epidemiology (Y.P. and N.J.S.); Mechanisms/pathophysiology (A.A.-K., A.C., A.B. and R.F.S.); Diagnosis, screening and prevention (Y.P., M.D.P., P.G.I. and R.C.F.); Management (Y.P., A.A.-K. and O.P.); Quality of life (Y.P.); Outlook (Y.P., A.A.-K. and P.D.S.); Overview of Primer (Y.P., A.A.-K. and P.D.S.).
Corresponding author
Ethics declarations
Competing interests
N.J.S. has received research grants from Medtronic, CSA Medical, C2 Therapeutics, CDx Medical, EndoStim and Interpace Diagnostics and has served as a consultant for Pfizer, Boston Scientific and Shire. A.C. has equity interest in and is a consultant for Lucid Diagnostics. R.F.S. has served as a consultant and receives research support from Ironwood Pharmaceuticals. M.D.P. has received educational grants from Olympus and Medtronic and has served as consultant for Medtronic. P.G.I. has received research funding from Exact Sciences, Pentax Medical, Symple Surgical, Nine Point Medical and Medtronic and has served as a consultant for Medtronic, Symple Surgical and CSA Medical. O.P. has a speaker honorarium from Olympus, Fujifilm, Medtronic, Boston Scientific and Cook. R.C.F. is named on patents related to the Cytosponge and associated assays, which have been licensed to Covidien (now Medtronic), and has served as a consultant to Medtronic. P.D.S. has received research funding from Pentax Medical, EndoStim, Yakult, OncoDNA and Ella-CS. Y.P., A.A.-K. and A.B. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Peters, Y., Al-Kaabi, A., Shaheen, N.J. et al. Barrett oesophagus. Nat Rev Dis Primers 5, 35 (2019). https://doi.org/10.1038/s41572-019-0086-z
Published:
DOI: https://doi.org/10.1038/s41572-019-0086-z
This article is cited by
-
The occurrence and development mechanisms of esophageal stricture: state of the art review
Journal of Translational Medicine (2024)
-
Stage II oesophageal carcinoma: peril in disguise associated with cellular reprogramming and oncogenesis regulated by pseudogenes
BMC Genomics (2024)
-
Decoding spatiotemporal transcriptional dynamics and epithelial fibroblast crosstalk during gastroesophageal junction development through single cell analysis
Nature Communications (2024)
-
Dutch, UK and US professionals’ perceptions of screening for Barrett’s esophagus and esophageal adenocarcinoma: a concept mapping study
BMC Cancer (2023)
-
Genomic signatures of past and present chromosomal instability in Barrett’s esophagus and early esophageal adenocarcinoma
Nature Communications (2023)