Abstract
Introduction
Perioperative complications associated with spinal fusion have been investigated steadily to reduce morbidity and mortality. Although there are several reports reviewing abdominal complications occurring with anterior spinal fusion, complications related to posterior spinal fusion (PSF) are rare. However, abdominal compartment syndrome (ACS) after PSF could be the most fatal and unpredictable complication in spinal surgery.
Case presentation
This 73-year-old man with body mass index (BMI) of 23.02, and surgical history of appendectomy 10 years prior complained of severe nausea and vomiting on the second postoperative day of L4–5 transforaminal lumbar interbody fusion (TLIF). By postoperative day 4, he presented with dyspnea and fever, and the first diagnostic impression suggested aspiration pneumonia due to vomiting. Physical examination revealed severe abdominal distention and tenderness to palpation at most of the abdomen. Computed tomography (CT) scan of abdomen and chest revealed left inguinal hernia of the small bowel with incarceration suggesting intra-abdominal hypertension (IAH), and multifocal peri-bronchial consolidation in both lungs, respectively. His respiratory symptoms progressed to respiratory failure, and he was finally mechanically ventilated in conjunction with antibiotics. After 2 weeks of intensive care, the patient’s symptom had improved, and finally he was transferred to a nursing facility.
Discussion
IAH and ACS rarely occur as abdominal complications of PSF. We suggest several risk factors including body mass index, abdominal surgical history, and long segment fusion for development of abdominal complications.
Similar content being viewed by others
Introduction
Posterior spinal fusion (PSF) is related to perioperative morbidity and mortality in the adult population [1, 2]. Risk factors, including advanced age, presence of comorbidity, and long segment fusion are associated with development of perioperative complications in PSF [3, 4]. Understanding of relevant risk factors is critical to minimize morbidity and mortality.
Abdominal complications such as iliac vein or artery laceration, ureter injury, splenic rupture, and incisional hernia have been reported to occur in conjunction with anterior spinal fusion [5]. Patients with history of abdominal or pelvic surgery are at a high risk of complications after anterior spine surgery [6]. However, few reports have described abdominal complications such as superior mesenteric artery syndrome [7, 8], acute pancreatitis [9], and abdominal compartment syndrome (ACS) [10,11,12,13] after the posterior approach. It has not been determined if abdominal surgical history increases abdominal complication risk after PSF. Recently, patients with ACS following PSF have been reported [12, 13]. ACS is one of the most unpredictable and fatal complications following PSF and is defined by elevated intra-abdominal pressure (IAP) > 20 mmHg with evidence of organ failure [14]. The authors present a patient with a history of abdominal surgery who experienced a left inguinal hernia after posterior lumbar fusion (PLF), showing features of ACS. The patient was informed that data concerning the case would be submitted for publication, and he provided consent.
Case presentation
History and presentation
A 73-year-old man with body mass index (BMI) of 23.02, medical history of hypertension, and surgical history of appendectomy 10 years prior presented with a 6-month history of low back, both buttocks, and leg pain, and he was experiencing progressive intermittent claudication. Preoperative X-ray (Fig. 1a) and magnetic resonance imaging (Fig. 1b, c) of the lumbar spine showed spondylolisthesis of L4 on L5 and spinal stenosis between L4 and L5. We performed L4–5 transforaminal lumbar interbody fusion with posterior screw fixation using the minimally invasive technique. Under general anesthesia, the patient was in the prone position with Jackson table. The operation time was 1.5 h, and there was no special event during operation. Anteroposterior and lateral lumbar spine X-ray imaging was performed on the first postoperative day showed acceptable positioning of the interbody cage and posterior instrumentation (Fig. 2a, b).
On the second postoperative day, the patient began to show nausea, vomiting, abdominal distention, and pain. He began to suffer from dyspnea and fever, by daybreak of postoperative day 4, then he was transferred from a private spine hospital to the advanced general institution’s emergency department for further evaluation.
Physical examination and investigations
On physical examination, he showed tenderness to palpation at the left upper quadrant, left lower quadrant, and right lower quadrant of the abdomen. There was no rebound tenderness or abdominal guarding. Laboratory studies revealed a white cell count of 13,310 per microliter (reference range: 4000–10,800), C-reactive protein (CRP) of 415.6 mg/L (reference range: 0.1–6.0), and procalcitonin of 5.66 ng/mL (reference range: 0.02–0.5). Arterial blood gas analysis revealed a pH of 7.395, pCO2 of 37.2 mmHg, and pO2 of 65.2 mmHg.
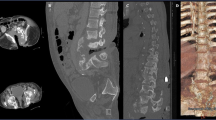
Abdominal and chest X-ray showed an enlarged abdomen with gas distention in the stomach (Fig. 3a) and pneumonia in both lungs (Fig. 3b). Abdominal computed tomography (CT) scan revealed left inguinal hernia of the small bowel with incarceration (Fig. 4a, b) and small pelvic ascites, but there was no sign of strangulation. Chest CT further showed multifocal peri-bronchial consolidation in both lungs with atelectasis in the right upper lung and both lower lungs (Fig. 4c).
Treatment
Because the patient presented with vomiting, fever, and dyspnea and there was evidence of pneumonia on chest X-ray, we assumed his diagnosis as aspiration pneumonia and treated him with antibiotics. We empirically started tazobactam, piperacillin, and ciprofloxacin, but his respiratory symptoms progressed to respiratory failure. He was admitted to the intensive care unit, intubated, and mechanically ventilated in conjunction with changed antibiotics (meropenem and levofloxacin). After about 2 weeks of mechanical ventilation, his symptoms and chest X-ray findings had improved. Finally, he was extubated and transferred to a nursing facility. CRP level was followed throughout his stay and revealed a prominent downward trend from 415.6 mg/L at presentation to 44.8 mg/L by 1-month postoperatively.
Although there was an incarcerated inguinal hernia of the small bowel with severe bowel distention suggesting IAH, there was no strangulation sign on CT finding. Nasogastric tube and rectal tube were inserted and maintained with negative pressure for 10 days. Three liters of greenish to dark brown color fluid drained through nasogastric tube for 2 days and decreased gradually afterwards. The inguinal hernia was manually reduced twice, and hernia truss was applied. Elective hernia surgery was scheduled later due to his general condition.
Discussion
Understanding complications and relevant risk factors after spinal fusion is important to minimize perioperative morbidity and mortality. It is well-known that pseudoarthrosis, wound infection, neural injury, dural tear, malpositioned screws, deep vein thrombosis, and arachnoiditis are complications of spinal fusion surgery [15, 16]. Czerwein et al. reported that abdominal complications such as vascular injury (iliac vein, artery, and inferior vena cava tear), visceral injury (ureteral injury, peritoneal tears, and bowel injury), and neural injury (injury to sympathetic chain, hypogastric plexus) can occur in conjunction with anterior lumbar fusion [5].
However, there is scant literature concerning abdominal complications after Posterior spinal fusion (PSF). We briefly summarize abdominal complications following anterior or Posterior lumbar fusion (PLF) (Table 1) [5,6,7, 9, 11]. Types of abdominal complications after PSF, include acute pancreatitis [9], superior mesenteric artery syndrome [7, 8], and Abdominal compartment syndrome (ACS) [10,11,12,13]. In this case, we report a patient who experienced inguinal hernia and developed significant abdominal pathology requiring intensive medical care following PLF. Recently, it was revealed that patients with a history of abdominal or pelvic surgery are at a higher risk of developing abdominal complications following anterior spinal fusion [6]. However, risk factors for developing ACS following PSF have not been determined.
In this paper, we review reported ACS cases after PSF and propose probable risk factors for ACS development following posterior spinal surgery. ACS is defined as sustained Intraabdominal pressure (IAP) greater than 20 mmHg concurrent with evidence of organ dysfunction [14]. To determine IAP, different techniques such as transbladder, uterus, rectum, stomach methods could be utilized [17]. Because all air needs to be aspirated from stomach, measurement of IAP through nasogastric tube is inconvenient [17]. Measurement of bladder pressure is commonly used as a standard screening tool for ACS or Intraabdominal hypertension (IAH) [18]. Although the pathophysiology of ACS is not well established, it has been revealed that elevated IAP causes regional blood flow disturbances affecting the lung, kidney, and heart, resulting in IAH-induced organ dysfunction and development of ACS [19]. When patient is diagnosed with ACS or IAH, IAP should be measured first every 4–6 h with medical treatment to reduce IAP. Medical treatments are focused on symptomatic treatment including sedation, nasogastric/colonic decompression, use of promotility agents, diuretics, and fluid resuscitation [18]. If patient’s IAH/ACS is refractory to medical management, decompressive laparotomy must be strongly considered. Although there are no specific diagnostic methods and therapies, the prognosis of ACS is relatively better when diagnosed early [20].
Recently, Holodinsky et al. [21] reported 16 risk factors associated with ACS and 25 with IAH including obesity, age, and postlaparotomy status. It is also well-known that prone position contributes to IAP elevation [22,23,24,25], and obese patients have higher IAP compared to normal BMI patients [26, 27]. Thus, we suggest that patients who undergo PSF are more prone to suffer from ACS complications due to the position compared with anterior spinal fusion. Among the various prone position, Jackson table is the best method to decrease IAP [28]. We used Jackson table, so operation position is not related with ACS in this case. Previous abdominal surgical history and BMI could be risk factors for developing IAH or ACS after PSF. In this case, the patient who was finally diagnosed with acute respiratory distress syndrome had severe abdominal distention with left incarcerated inguinal hernia, small pelvic ascites, minimal pericardial effusion, and multifocal peribronchial consolidation. Although we did not measure intravesical pressure, we assume that the patient was experiencing IAH, because organ dysfunction exists at IAP levels between 12 and 20 mmHg—the range defined as IAH [29].
To our knowledge, eight cases have been reported for ACS or IAH after PSF [10,11,12,13] (Table 2). Almost all the studies clinically diagnosed patients with ACS or IAH without any measurement of bladder pressure. Most of patients presented with typical abdominal symptoms such as severe abdominal pain, nausea and vomiting in conjunction with CT findings, including peritonitis, ileus and inguinal hernia. Because our case experienced severe abdominal pain and vomiting with findings of inguinal hernia, peribronchial pneumonia and pelvic ascites in the setting of increased IAP, we also diagnosed our patient with IAH, which diagnostic process is consistent with previous reports. Thus, we infer that the diagnosis of ACS or IAH after PSF should be considered in the clinical experience. We found that most cases of spine surgery were long segment fusion, which require a long operation time in the prone position. Shih et al. [12] also reported four patients with high BMI and abdominal surgical history who developed ACS after PSF. We suggest that long segments fusion, abdominal surgical history and BMI could be the risk factor for development of ACS/IAH after PSF considering the number of previous cases. However, risk factors like obesity and long segments fusion, did not match in this case. To know the exact risk factors, we need further study including more cases.
The risk factors of IAH is still unclear, but if there is any sign suggesting bowel obstruction such as increased nausea, vomiting, and worsening abdominal pain, high suspicion for IAH is required. Physical examination to assess distended abdomen, plain radiography, and CT scanning of the abdomen and pelvis should be performed quickly for early detection of ACS, which might require surgical decompression if refractory to medical management. Measurement of bladder pressure can be utilized to determine IAP. Furthermore, along with several suggested risk factors including abdominal surgical history, high BMI and long segments surgery, patients who undergo PSF should be carefully monitored for development of ACS or IAH in the intraoperative and postoperative periods.
Conclusion
Complications of spinal fusion contribute to perioperative morbidity and mortality. Therefore, it is essential to understand predisposing risk factors and minimize complications. Abdominal complications such as ACS rarely occur in conjunction with PSF but are associated with significant morbidity and mortality. If patients who have several suggested risk factors complain of severe nausea or vomiting after PSF, it is critical to suspect ACS or IAH as early detection and intervention are imperative.
References
Ma Y, Passias P, Gaber-Baylis LK, Girardi FP, Memtsoudis SG. Comparative in-hospital morbidity and mortality after revision versus primary thoracic and lumbar spine fusion. Spine J. 2010;10:881–9.
Memtsoudis SG, Vougioukas VI, Ma Y, Gaber-Baylis LK, Girardi FP. Perioperative morbidity and mortality after anterior, posterior, and anterior/posterior spine fusion surgery. Spine. 2011;36:1867–77.
Cassinelli EH, Eubanks J, Vogt M, Furey C, Yoo J, Bohlman HH. Risk factors for the development of perioperative complications in elderly patients undergoing lumbar decompression and arthrodesis for spinal stenosis: an analysis of 166 patients. Spine. 2007;32:230–5.
Zheng F, Cammisa FP Jr., Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine. 2002;27:818–24.
Czerwein JK Jr., Thakur N, Migliori SJ, Lucas P, Palumbo M. Complications of anterior lumbar surgery. J Am Acad Orthop Surg. 2011;19:251–8.
Osler P, Kim SD, Hess KA, Phan P, Simpson AK, Mansfield FL, et al. Prior abdominal surgery is associated with an increased risk of postoperative complications after anterior lumbar interbody fusion. Spine. 2014;39:E650–6.
Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27:E528–33.
Derincek A, Wood KB, Muench CA. Superior mesenteric artery syndrome following correction of kyphosis in an adult. J Spinal Disord Tech. 2004;17:549–53.
Kobayashi K, Imagama S, Ito Z, Ando K, Shinjo R, Yagi H, et al. Hyperamylasemia and pancreatitis following posterior spinal surgery. J Orthop Sci. 2015;20:967–72.
Boniello A, Verma K, Sees JP, Miller F, Dabney K. Delayed abdominal compartment syndrome as a complication of spinal surgery: literature review and case report. Spine Deform. 2013;1:464–7.
Cravero JP, Muffly M. Case presentation: abdominal compartment syndrome complicating posterior spinal fusion. Paediatr Anaesth. 2012;22:278–80.
Shih P, Slimack NP, Roy A, Fessler RG, Koski TR. Abdominal complications following posterior spinal fusion in patients with previous abdominal surgeries. Neurosurg Focus. 2011;31:E16.
Sugrue PA, O’Shaughnessy BA, Nasr F, Koski TR, Ondra SL. Abdominal complications following kyphosis correction in ankylosing spondylitis. J Neurosurg Spine. 2009;10:154–9.
Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–32.
Davne SH, Myers DL. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine. 1992;17:S184–9.
Hee HT, Castro FP Jr., Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14:533–40.
Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004;30:357–71.
Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–206.
Cheatham ML. Abdominal compartment syndrome: pathophysiology and definitions. Scand J Trauma Resusc Emerg Med. 2009;17:10.
Nguyen J, Noory M, Capano-Wehrle L, Gaughan J, Hazelton JP. Expeditious diagnosis and laparotomy for patients with acute abdominal compartment syndrome may improve survival. Am Surg. 2018;84:1836–40.
Holodinsky JK, Roberts DJ, Ball CG, Blaser AR, Starkopf J, Zygun DA, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013;17:R249.
Chiumello D, Cressoni M, Racagni M, Landi L, Li Bassi G, Polli F, et al. Effects of thoraco-pelvic supports during prone position in patients with acute lung injury/acute respiratory distress syndrome: a physiological study. Crit Care. 2006;10:R87.
Hering R, Vorwerk R, Wrigge H, Zinserling J, Schroder S, von Spiegel T, et al. Prone positioning, systemic hemodynamics, hepatic indocyanine green kinetics, and gastric intramucosal energy balance in patients with acute lung injury. Intensive Care Med. 2002;28:53–8.
Park CK. The effect of patient positioning on intraabdominal pressure and blood loss in spinal surgery. Anesth Analg. 2000;91:552–7.
Han IH, Son DW, Nam KH, Choi BK, Song GS. The effect of body mass index on intra-abdominal pressure and blood loss in lumbar spine surgery. J Korean Neurosurg Soc. 2012;51:81–5.
Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg. 2005;15:1225–32.
Wilson A, Longhi J, Goldman C, McNatt S. Intra-abdominal pressure and the morbidly obese patients: the effect of body mass index. J Trauma. 2010;69:78–83.
Kim E, Kim HC, Lim YJ, Kim CH, Sohn S, Chung CK, et al. Comparison of intra-abdominal pressure among 3 prone positional apparatuses after changing from the supine to the prone position and applying positive end-expiratory pressure in healthy euvolemic patients: a prospective observational study. J Neurosurg Anesthesiol. 2017;29:14–20.
De Waele JJ, De Laet I, Malbrain ML. Understanding abdominal compartment syndrome. Intensive Care Med. 2016;42:1068–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oh, HC., Kim, HS. & Park, JY. Abdominal compartment syndrome following posterior lumbar fusion in a patient with previous abdominal surgery. Spinal Cord Ser Cases 5, 47 (2019). https://doi.org/10.1038/s41394-019-0191-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-019-0191-y