Abstract
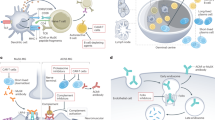
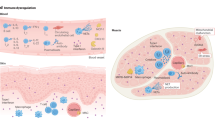
Myasthenia gravis (MG) is an autoimmune disease caused by antibodies against the acetylcholine receptor (AChR), muscle-specific kinase (MuSK) or other AChR-related proteins in the postsynaptic muscle membrane. Localized or general muscle weakness is the predominant symptom and is induced by the antibodies. Patients are grouped according to the presence of antibodies, symptoms, age at onset and thymus pathology. Diagnosis is straightforward in most patients with typical symptoms and a positive antibody test, although a detailed clinical and neurophysiological examination is important in antibody-negative patients. MG therapy should be ambitious and aim for clinical remission or only mild symptoms with near-normal function and quality of life. Treatment should be based on MG subgroup and includes symptomatic treatment using acetylcholinesterase inhibitors, thymectomy and immunotherapy. Intravenous immunoglobulin and plasma exchange are fast-acting treatments used for disease exacerbations, and intensive care is necessary during exacerbations with respiratory failure. Comorbidity is frequent, particularly in elderly patients. Active physical training should be encouraged.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gilhus, N. E. Myasthenia gravis. N. Engl. J. Med. 375, 2570–2581 (2016).
Gilhus, N. E. & Verschuuren, J. J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 14, 1023–1036 (2015). This review article covers most MG aspects.
Kerty, E., Elsais, A., Argov, Z., Evoli, A. & Gilhus, N. E. EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur. J. Neurol. 21, 687–693 (2014).
Al-bassam, W. et al. Characteristics, incidence, and outcome of patients admitted to the intensive care unit with myasthenia gravis. J. Crit. Care 45, 90–94 (2018).
Andersen, J. B., Gilhus, N. E. & Sanders, D. B. Factors affecting outcome in myasthenia gravis. Muscle Nerve 54, 1041–1049 (2016).
Hong, Y. et al. Multiple antibody detection in ‘seronegative’ myasthenia gravis patients. Eur. J. Neurol. 24, 844–850 (2017).
Marx, A. et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun. Rev. 12, 875–884 (2013).
Carr, A. S., Cardwell, C. R., McCarron, P. O. & McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 10, 46 (2010).
Park, S. Y., Lee, J. Y., Lim, N. G. & Hong, Y. H. Incidence and prevalence of myasthenia gravis in Korea: a population-based study using the National Health Insurance Claims Database. J. Clin. Neurol. 12, 340–344 (2016).
Heldal, A. T., Owe, J. F., Gilhus, N. E. & Romi, F. Seropositive myasthenia gravis: a nationwide epidemiologic study. Neurology 73, 150–151 (2009).
Zieda, A. et al. A nationwide epidemiological study of myasthenia gravis in Latvia. Eur. J. Neurol. 25, 519–526 (2018).
Andersen, J. B., Heldal, A. T., Engeland, A. & Gilhus, N. E. Myasthenia gravis epidemiology in a national cohort; combining multiple disease registries. Acta Neurol. Scand. 129, 26–31 (2014).
Hong, Y. et al. Juvenile-onset myasthenia gravis: autoantibody status, clinical characteristics and genetic polymorphisms. J. Neurol. 264, 955–962 (2017).
Matsuki, K. et al. HLA antigens in Japanese patients with myasthenia-gravis. J. Clin. Invest. 86, 392–399 (1990).
Matsuki, K., Maeda, H., Nomura, Y. & Segawa, M. Distortion of HLA gene transmission in childhood-onset myasthenia-gravis. Lancet 340, 796–796 (1992).
Popperud, T. H., Boldingh, M. I., Rasmussen, M. & Kerty, E. Juvenile myasthenia gravis in Norway: clinical characteristics, treatment, and long-term outcome in a nationwide population-based cohort. Eur. J. Paediatr. Neurol. 21, 707–714 (2017).
Evoli, A. et al. Myasthenia gravis with antibodies to MuSK: an update. Ann. NY Acad. Sci. 1412, 82–89 (2018). This article represents a comprehensive review of an important MG subgroup.
Hong, Y. et al. Autoantibody profile and clinical characteristics in a cohort of Chinese adult myasthenia gravis patients. J. Neuroimmunol. 298, 51–57 (2016).
Hong, Y., Li, H. F., Romi, F., Skeie, G. O. & Gilhus, N. E. HLA and MuSK-positive myasthenia gravis: a systemic review and meta-analysis. Acta Neurol. Scand. 138, 219–226 (2018).
Huda, S., Woodhall, M. R., Vincent, A. & Heckmann, J. M. Characteristics of acetylcholine-receptor-antibody-negative myasthenia gravis in a South African cohort. Muscle Nerve 54, 1023–1029 (2016).
Zisimopoulou, P. et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J. Autoimmun. 52, 139–145 (2014). This large, multinational study establishes the presence, frequency and some clinical characteristics of anti-LRP4 MG.
Yan, M., Xing, G. L., Xiong, W. C. & Mei, L. Agrin and LRP4 antibodies as new biomarkers of myasthenia gravis. Ann. NY Acad. Sci. 1413, 126–135 (2018).
Zhang, B. et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch. Neurol. 69, 445–451 (2012).
Boldingh, M. I. et al. Prevalence and clinical aspects of immigrants with myasthenia gravis in Northern Europe. Muscle Nerve 55, 819–827 (2017).
Owe, J. F., Daltveit, A. K. & Gilhus, N. E. Causes of death among patients with myasthenia gravis in Norway between 1951 and 2001. J. Neurol. Neurosurg. Psychiatry 77, 203–207 (2006).
Hansen, J. S. et al. Mortality in myasthenia gravis: a nationwide population-based follow-up study in Denmark. Muscle Nerve 53, 73–77 (2016). This population-based registry study proves the excellent prognosis of MG with modern treatment.
Gilhus, N. E., Nacu, A., Andersen, J. B. & Owe, J. F. Myasthenia gravis and risks for comorbidity. Eur. J. Neurol. 22, 17–23 (2015).
Ogawa, Y. et al. Survey of epidemiology, clinical picture and current treatments for elderly-onset (>=65 years) patients with myasthenia gravis in Nagano Prefecture. Japan. Neurol. Clin. Neurosci. 5, 107–112 (2017).
Pedersen, E. G., Hallas, J., Hansen, K., Jensen, P. E. H. & Gaist, D. Late-onset myasthenia not on the increase: a nationwide register study in Denmark, 1996–2009. Eur. J. Neurol. 20, 309–314 (2013).
Boldingh, M. I. et al. Geographical distribution of myasthenia gravis in Northern Europe - results from a population-based study from two countries. Neuroepidemiology 44, 221–231 (2015).
Cea, G. et al. Clinical and epidemiological features of myasthenia gravis in Chilean population. Acta Neurol. Scand. 138, 338–343 (2018).
Ramanujam, R., Pirskanen, R., Ramanujam, S. & Hammarstrom, L. Utilizing twins concordance rates to infer the predisposition to myasthenia gravis. Twin Res. Hum. Genet. 14, 129–136 (2011).
Li, F., Yuan, W. Z. & Wu, X. S. Association of CTLA-4 polymorphisms with increased risks of myasthenia gravis. Ann. Hum. Genet. 82, 358–369 (2018).
Giraud, M. et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 448, 934–937 (2007).
Punga, A. R. & Punga, T. Circulating microRNAs as potential biomarkers in myasthenia gravis patients. Ann. NY Acad. Sci. 1412, 33–40 (2018).
Boldingh, M. I. et al. Increased risk for clinical onset of myasthenia gravis during the postpartum period. Neurology 87, 2139–2145 (2016).
Berrih-Aknin, S. & Le Panse, R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J. Autoimmun. 52, 90–100 (2014). This article explains the major immunological mechanisms in MG.
Dragin, N. et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Invest. 126, 1525–1537 (2016).
Cavalcante, P. et al. Epstein-barr virus persistence and reactivation in myasthenia gravis thymus. Ann. Neurol. 67, 726–738 (2010).
Meyer, M. et al. Lack of evidence for epstein-barr virus infection in myasthenia gravis thymus. Ann. Neurol. 70, 515–518 (2011).
Gilhus, N. E., Romi, F., Hong, Y. & Skeie, G. O. Myasthenia gravis and infectious disease. J. Neurol. 265, 1251–1258 (2018).
Kao, J. C., Brickshawana, A. & Liewluck, T. Neuromuscular complications of programmed cell death-1 (PD-1) inhibitors. Curr. Neurol. Neurosci. Rep. 18, 63 (2018).
Benfaremo, D., Manfredi, L., Luchetti, M. M. & Gabrielli, A. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: a review of the literature. Curr. Drug Safety 13, 150–164 (2018).
Patrick, J. & Lindstrom, J. Autoimmune response to acetylcholine receptor. Science 180, 871–872 (1973).
Lindstrom, J. M., Seybold, M. E., Lennon, V. A., Whittingham, S. & Duane, D. D. Antibody to acetylcholine-receptor in myasthenia-gravis - prevalence, clinical correlates, and diagnostic value. Neurology 26, 1054–1059 (1976).
Toyka, K. V., Drachman, D. B., Pestronk, A. & Kao, I. Myasthenia-gravis - passive transfer from man to mouse. Science 190, 397–399 (1975).
Zisimopoulou, P., Brenner, T., Trakas, N. & Tzartos, S. J. Serological diagnostics in myasthenia gravis based on novel assays and recently identified antigens. Autoimmun. Rev. 12, 924–930 (2013).
Changeux, J. P. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem. 287, 40207–40215 (2012).
Luo, J. et al. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J. Neurosci. 29, 13898–13908 (2009).
Tzartos, S. J. et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol. Rev. 163, 89–120 (1998).
Noridomi, K., Watanabe, G., Hansen, M. N., Han, G. W. & Chen, L. Structural insights into the molecular mechanisms of myasthenia gravis and their therapeutic implications. eLife 6, e23043 (2017). This study clarifies the pathogenetic effect of anti-AChR antibodies.
Tuzun, E. & Christadoss, P. Complement associated pathogenic mechanisms in myasthenia gravis. Autoimmun. Rev. 12, 904–911 (2013).
Kordas, G., Lagoumintzis, G., Sideris, S., Poulas, K. & Tzartos, S. J. Direct proof of the in vivo pathogenic role of the AChR autoantibodies from myasthenia gravis patients. PLOS ONE 10, e0117673 (2015).
Gilhus, N. E. et al. Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 12, 259–268 (2016).
Hara, H. et al. Detection and characterization of blocking-type antiacetylcholine receptor antibodies in sera from patients with myasthenia-gravis. Clin. Chem. 39, 2053–2057 (1993).
Messeant, J. et al. MuSK frizzled-like domain is critical for mammalian neuromuscular junction formation and maintenance. J. Neurosci. 35, 4926–4941 (2015).
Li, L. et al. Enzymatic activity of the scaffold protein rapsyn for synapse formation. Neuron 92, 1007–1019 (2016).
Hoch, W. et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 7, 365–368 (2001).
Koneczny, I. et al. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J. Autoimmun. 77, 104–115 (2017).
Huijbers, M. G. et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc. Natl Acad. Sci. USA 110, 20783–20788 (2013). This study represents an achievement in understanding the role of anti-MuSK antibodies in disease development.
Huijbers, M. G. et al. Longitudinal epitope mapping in MuSK myasthenia gravis: implications for disease severity. J. Neuroimmunol. 291, 82–88 (2016).
Poulas, K., Koutsouraki, E., Kordas, G., Kokla, A. & Tzartos, S. J. Anti-MuSK- and anti-AChR-positive myasthenia gravis induced by d-penicillamine. J. Neuroimmunol. 250, 94–98 (2012).
Kim, N. et al. Lrp4 is a receptor for agrin and forms a complex with MuSK. Cell 135, 334–342 (2008).
Higuchi, O., Hamuro, J., Motomura, M. & Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann. Neurol. 69, 418–422 (2011).
Tzartos, J. S. et al. LRP4 antibodies in serum and CSF from amyotrophic lateral sclerosis patients. Ann. Clin. Transl Neurol. 1, 80–87 (2014).
Shen, C. Y. et al. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J. Clin. Invest. 123, 5190–5202 (2013).
Ulusoy, C., Cavus, F., Yilmaz, V. & Tuzun, E. Immunization with recombinantly expressed LRP4 induces experimental autoimmune myasthenia gravis in C57BL/6 mice. Immunol. Invest. 46, 490–499 (2017).
Mori, S. et al. Immunization of mice with LRP4 induces myasthenia similar to MuSK-associated myasthenia gravis. Exp. Neurol. 297, 158–167 (2017).
Witzemann, V., Chevessier, F., Pacifici, P. G. & Yampolsky, P. The neuromuscular junction: selective remodeling of synaptic regulators at the nerve/muscle interface. Mech. Dev. 130, 402–411 (2013).
Gasperi, C. et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology 82, 1976–1983 (2014).
Yan, M. et al. Induction of anti-agrin antibodies causes myasthenia gravis in mice. Neuroscience 373, 113–121 (2018).
Cossins, J. et al. in Myasthenia Gravis and Related Disorders II: 12th International Conference Vol. 1275 (eds Wolfe, G. I., Meriggioli, M. N., Ciafaloni, E. & Ruff, R. L.) 123–128 (Wiley, 2012).
Romi, F. et al. Anti-voltage-gated potassium channel Kv1.4 antibodies in myasthenia gravis. J. Neurol. 259, 1312–1316 (2012).
Suzuki, S. et al. Cardiac involvements in myasthenia gravis associated with anti-Kv1.4 antibodies. Eur. J. Neurol. 21, 223–230 (2014).
O’Connell, K. M. S., Whitesell, J. D. & Tamkun, M. M. Localization and mobility of the delayed-rectifer K+ channel Kv2.1 in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 294, H229–H237 (2008).
Powers, K. et al. Titin force enhancement following active stretch of skinned skeletal muscle fibres. J. Exp. Biol. 220, 3110–3118 (2017).
Romi, F., Hong, Y. & Gilhus, N. E. Pathophysiology and immunological profile of myasthenia gravis and its subgroups. Curr. Opin. Immunol. 49, 9–13 (2017).
Szczudlik, P. et al. Antititin antibody in early- and late-onset myasthenia gravis. Acta Neurol. Scand. 130, 229–233 (2014).
Gautel, M. et al. Titin antibodies in myasthenia-gravis - identification of a major immunogenic region of titin. Neurology 43, 1581–1585 (1993).
Santulli, G., Nakashima, R., Yuan, Q. & Marks, A. R. Intracellular calcium release channels: an update. J. Physiol. 595, 3041–3051 (2017).
Mygland, A. et al. Ryanodine receptor autoantibodies in myasthenia-gravis patients with a thymoma. Ann. Neurol. 32, 589–591 (1992).
Romi, F., Skeie, G. O., Gilhus, N. E. & Aarli, J. A. Striational antibodies in myasthenia gravis - Reactivity and possible clinical significance. Arch. Neurol. 62, 442–446 (2005).
Gallardo, E. et al. Cortactin autoantibodies in myasthenia gravis. Autoimmun. Rev. 13, 1003–1007 (2014).
Illa, I., Cortes-Vicente, E., Martinez, M. A. & Gallardo, E. Diagnostic utility of cortactin antibodies in myasthenia gravis. Ann. NY Acad. Sci. 1412, 90–94 (2018).
Labrador-Horrillo, M. et al. Identification of a novel myositis-associated antibody directed against cortactin. Autoimmun. Rev. 13, 1008–1012 (2014).
Strobel, P. et al. The ageing and myasthenic thymus: a morphometric study validating a standard procedure in the histological workup of thymic specimens. J. Neuroimmunol. 201, 64–73 (2008).
Wolfe, G. I. et al. Randomized trial of thymectomy in myasthenia gravis. N. Engl. J. Med. 375, 511–522 (2016). This randomized trial confirms the efficacy of thymectomy in some MG subgroups with anti-AChR antibodies.
Cron, M. A. et al. Thymus involvement in early-onset myasthenia gravis. Ann. NY Acad. Sci. 1412, 137–145 (2018).
Valencia, X. & Lipsky, P. E. CD4+ CD25+ FoxP3+ regulatory T cells in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 3, 619–626 (2007).
Cheng, M. & Anderson, M. S. Thymic tolerance as a key brake on autoimmunity. Nat. Immunol. 19, 659–664 (2018).
Weis, C. A., Schalke, B., Strobel, P. & Marx, A. Challenging the current model of early-onset myasthenia gravis pathogenesis in the light of the MGTX trial and histological heterogeneity of thymectomy specimens. Ann. NY Acad. Sci. 1413, 82–91 (2018).
Yi, J. S. et al. Characterization of CD4 and CD8 T cell responses in MuSK myasthenia gravis. J. Autoimmun. 52, 130–138 (2014).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010).
Luther, C. et al. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J. Immunol. 183, 841–848 (2009).
Gradolatto, A. et al. Both Treg cells and Tconv cells are defective in the myasthenia gravis thymus: roles of IL-17 and TNF-alpha. J. Autoimmun. 52, 53–63 (2014).
Marx, A. et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp. Neurol. 270, 55–65 (2015).
Leite, M. I. et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann. Neurol. 57, 444–448 (2005).
Singman, E. L., Matta, N. S. & Silbert, D. I. Use of the Cogan lid twitch to identify myasthenia gravis. J. Neuroophthalmol. 31, 239–240 (2011).
Van Stavern, G. P., Bhatt, A., Haviland, J. & Black, E. H. A prospective study assessing the utility of Cogan’s lid twitch sign in patients with isolated unilateral or bilateral ptosis. J. Neurol. Sci. 256, 84–85 (2007).
Chatzistefanou, K. I. et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology 116, 2236–2243 (2009).
Yamamoto, D. et al. Effect of local cooling on excitation-contraction coupling in myasthenic muscle: another mechanism of ice-pack test in myasthenia gravis. Clin. Neurophysiol. 128, 2309–2317 (2017).
Richards, J. & Howard, J. F. Seronegative myasthenia gravis associated with malignant thymoma. Neuromuscul. Disord. 27, 417–418 (2017).
Stergiou, C. et al. Titin antibodies in “seronegative” myasthenia gravis - a new role for an old antigen. J. Neuroimmunol. 292, 108–115 (2016).
Leite, M. I. et al. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain 131, 1940–1952 (2008). This study discovers that some MG autoantibodies are undetectable by the classical methods and introduces cell-based assays.
Cruz, P. M. R. et al. Clinical features and diagnostic usefulness of antibodies to clustered acetylcholine receptors in the diagnosis of seronegative myasthenia gravis. JAMA Neurol. 72, 642–649 (2015). This study reports the characteristics of all patients with anti-AChR antibodies exclusively identified by the cell-based assay.
Vincent, A. et al. in Myasthenia Gravis and Related Disorders: 11th International Conference Vol. 1132 (eds Kaminski, H. J. & Barohn, R. J.) 84–92 (The New York Academy of Sciences, 2008).
Tsonis, A. I. et al. MuSK autoantibodies in myasthenia gravis detected by cell based assay - a multinational study. J. Neuroimmunol. 284, 10–17 (2015).
Huda, S. et al. IgG-specific cell-based assay detects potentially pathogenic MuSK-Abs in seronegative MG. Neurol. Neuroimmunol. Neuroinflamm. 4, e357 (2017).
Liik, M. & Punga, A. R. Repetitive nerve stimulation often fails to detect abnormal decrement in acute severe generalized myasthenia gravis. Clin. Neurophysiol. 127, 3480–3484 (2016).
Nikolic, A., Basta, I., Stojanovic, V. R., Stevic, Z. & Lavrnic, D. Electrophysiological profile of the patients with MuSK positive myasthenia gravis. Neurol. Res. 36, 945–949 (2014).
Nikolic, A. V., Bojic, S. D., Stojanovic, V. M. R., Basta, I. Z. & Lavrnic, D. V. Electrophysiological findings in patients with low density lipoprotein receptor related protein 4 positive myasthenia gravis. Eur. J. Neurol. 23, 1635–1641 (2016).
Stalberg, E., Sanders, D. B. & Kouyoumdjian, J. A. Pitfalls and errors in measuring jitter. Clin. Neurophysiol. 128, 2233–2241 (2017). This article informs about how to interpret key neurophysiological findings in MG.
Thornton, R. C. & Michell, A. W. Techniques and applications of EMG: measuring motor units from structure to function. J. Neurol. 259, 585–594 (2012).
Cruz, P. M. R., Palace, J. & Beeson, D. The neuromuscular junction and wide heterogeneity of congenital myasthenic syndromes. Int. J. Mol. Sci. 19, E1677 (2018).
Lee, M., Beeson, D. & Palace, J. Therapeutic strategies for congenital myasthenic syndromes. Ann. NY Acad. Sci. 1412, 129–136 (2018).
Titulaer, M. J., Lang, B. & Verschuuren, J. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 10, 1098–1107 (2011).
Verschuuren, J., Strijbos, E. & Vincent, A. Neuromuscular junction disorders. Handb. Clin. Neurol. 133, 447–466 (2016).
Sanders, D. B. et al. Is the decremental pattern in Lambert-Eaton syndrome different from that in myasthenia gravis? Clin. Neurophysiol. 125, 1274–1277 (2014).
Di Lorenzo, R. et al. Low specificity of voltage-gated calcium channel antibodies in Lambert-Eaton myasthenic syndrome: a call for caution. J. Neurol. 265, 2114–2119 (2018).
Lacey, B., Chang, W. & Rootman, J. Nonthyroid causes of extraocular muscle disease. Surv. Ophthalmol. 44, 187–213 (1999).
Skeie, G. O. et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur. J. Neurol. 17, 893–902 (2010).
Sanders, D. B. et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 87, 419–425 (2016).
Sussman, J. et al. The Association of British Neurologists’ myasthenia gravis guidelines. Ann. NY Acad. Sci. 1412, 166–169 (2018).
Murai, H. et al. Rationale for the clinical guidelines for myasthenia gravis in Japan. Ann. NY Acad. Sci. 1413, 35–40 (2018).
Pitha, J. et al. Guideline for the diagnosis and therapy of myasthenia gravis. Cesk. Neurol. Neurochir. 75, 244–252 (2012).
Gilhus, N. E., Kerty, E., Loseth, S., Mygland, A. & Tallaksen, C. Myasthenia gravis - diagnosis and treatment [Norwegian]. Tidsskr. Nor. Laegeforen. 136, 1089–1094 (2016).
Evoli, A. & Padua, L. Diagnosis and therapy of myasthenia gravis with antibodies to muscle-specific kinase. Autoimmun. Rev. 12, 931–935 (2013).
Bonanno, S. et al. Amifampridine phosphate in the treatment of muscle-specific kinase myasthenia gravis: a phase IIb, randomized, double-blind, placebo-controlled, double crossover study. Sage Open Med. 6, 2050312118819013 (2018).
Mantegazza, R., Bonanno, S., Camera, G. & Antozzi, C. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr. Dis. Treat 7, 151–160 (2011).
Meriggioli, M. N. & Sanders, D. B. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 8, 475–490 (2009).
Sieb, J. P. Myasthenia gravis: an update for the clinician. Clin. Exp. Immunol. 175, 408–418 (2014).
Sanders, D. B. & Evoli, A. Immunosuppressive therapies in myasthenia gravis. Autoimmunity 43, 428–435 (2010).
Utsugisawa, K. et al. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve 50, 493–500 (2014).
Gotterer, L. & Li, Y. B. Maintenance immunosuppression in myasthenia gravis. J. Neurol. Sci. 369, 294–302 (2016).
Benatar, M. et al. Efficacy of prednisone for the treatment of ocular myasthenia (epitome): a randomized, controlled trial. Muscle Nerve 53, 363–369 (2016).
El-Salem, K. et al. Treatment of MuSK-associated myasthenia gravis. Curr. Treat Options Neurol. 16, 283 (2014).
Hobson-Webb, L. D. et al. Can mycophenolate mofetil be tapered safely in myasthenia gravis? A retrospective, multicenter analysis. Muscle Nerve 52, 211–215 (2015).
Sathasivam, S. Current and emerging treatments for the management of myasthenia gravis. Ther. Clin. Risk Manag. 7, 313–323 (2011).
Hehir, M. K. et al. Mycophenolate mofetil in AChR-antibody-positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve 41, 593–598 (2010).
Cruz, J. L., Wolff, M. L., Vanderman, A. J. & Brown, J. N. The emerging role of tacrolimus in myasthenia gravis. Ther. Adv. Neurol. Disord. 8, 92–103 (2015).
Tindall, R. S. A., Phillips, J. T., Rollins, J. A., Wells, L. & Hall, K. A. Clinical therapeutic trial of cyclosporine in myasthenia-gravis. Ann. NY Acad. Sci. 681, 539–551 (1993).
Palace, J., Newsom-Davis, J. & Lecky, B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology 50, 1778–1783 (1998). This study still represents the scientific proof for the effect of combination immunosuppressive therapy in MG.
Sanders, D. B. et al. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology 71, 394–399 (2008).
Sanders, D. B. et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 71, 400–406 (2008).
Nagane, Y., Utsugisawa, K., Obara, D., Kondoh, R. & Terayama, Y. Efficacy of low-dose FK506 in the treatment of myasthenia gravis - a randomized pilot study. Eur. Neurol. 53, 146–150 (2005).
Yoshikawa, H., Kiuchi, T., Saida, T. & Takamori, M. Randomised, double-blind, placebo-controlled study of tacrolimus in myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 82, 970–977 (2011).
Pasnoor, M. et al. A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 87, 57–64 (2016).
Heckmann, J. M., Rawoot, A., Bateman, K., Renison, R. & Badri, M. A single-blinded trial of methotrexate versus azathioprine as steroid-sparing agents in generalized myasthenia gravis. BMC Neurol. 11, 97 (2011).
Tandan, R., Hehir, M. K., Waheed, W. & Howard, D. B. Rituximab treatment of myasthenia gravis: a systematic review. Muscle Nerve 56, 185–196 (2017). This article reviews the evidence thus far for rituximab as a promising therapy in MG.
Robeson, K. R. et al. Durability of the rituximab response in acetylcholine receptor autoantibody-positive myasthenia gravis. JAMA Neurol. 74, 60–66 (2017).
Anderson, D., Phan, C., Johnston, W. S. & Siddiqi, Z. A. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann. Clin. Transl Neurol. 3, 552–555 (2016).
Kanth, K. M., Solorzano, G. E. & Goldman, M. D. PML in a patient with myasthenia gravis treated with multiple immunosuppressing agents. Neurol. Clin. Pract. 6, E17–E19 (2016).
Afanasiev, V. et al. Resistant myasthenia gravis and rituximab: a monocentric retrospective study of 28 patients. Neuromuscul. Disord. 27, 251–258 (2017).
Howard, J. F. et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 16, 976–986 (2017). This article reports the promising results regarding the effect of a complement inhibitory drug in MG.
Gilhus, N. E. Eculizumab: a treatment option for mysthenia gravis? Lancet Neurol. 16, 947–948 (2017).
Hewett, K. et al. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology 90, E1425–E1434 (2018).
De Feo, L. G., Schottlender, J., Martelli, N. A. & Molfino, N. A. Use of intravenous pulsed cyclophosphamide in severe, generalized myasthenia gravis. Muscle Nerve 26, 31–36 (2002).
Drachman, D. B., Adams, R. N., Hu, R., Jones, R. J. & Brodsky, R. A. in Myasthenia Gravis and Related Disorders: 11th International Conference Vol. 1132 (eds Kaminski, H. J. & Barohn, R. J.) 305–314 (The New York Academy of Sciences, 2008).
Bryant, A. et al. Myasthenia gravis treated with autologous hematopoietic stem cell transplantation. JAMA Neurol. 73, 652–658 (2016).
Gelfand, E. W. Mechanisms of disease intravenous immune globulin in autoimmune and inflammatory diseases. N. Engl. J. Med. 367, 2015–2025 (2012).
Gajdos, P., Chevret, S., Clair, B., Tranchant, C. & Chastang, C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann. Neurol. 41, 789–796 (1997).
Gajdos, P., Chevret, S. & Toyka, K. V. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst. Rev. 12, CD002277 (2012).
Bourque, P. R., Pringle, C. E., Cameron, W., Cowan, J. & Chardon, J. W. Subcutaneous immunoglobulin therapy in the chronic management of myasthenia gravis: a retrospective cohort study. PLOS ONE 11, e0159993 (2016).
Beecher, G., Anderson, D. & Siddiqi, Z. A. Subcutaneous immunoglobulin in myasthenia gravis exacerbation: A prospective, open-label trial. Neurology 89, 1135–1141 (2017).
Meyer, D. M. et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann. Thorac Surg. 87, 385–391 (2009).
Keijzers, M. et al. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur. J. Cardiothorac Surg. 48, 40–45 (2015).
Gronseth, G. S. & Barohn, R. J. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review) - report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 55, 7–15 (2000).
Rahbek, M. A. et al. Exercise in myasthenia gravis: a feasibility study of aerobic and resistance training. Muscle Nerve 56, 700–709 (2017).
Westerberg, E., Molin, C. J., Lindblad, I., Emtner, M. & Punga, A. R. Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: a pilot study. Muscle Nerve 56, 207–214 (2017).
Nicolle, M. W. et al. Sleep apnea in patients with myasthenia gravis. Neurology 67, 140–142 (2006).
Pedersen, E. G. et al. Risk of non-melanoma skin cancer in myasthenia patients treated with azathioprine. Eur. J. Neurol. 21, 454–458 (2014).
Pedersen, E. G. et al. Myasthenia and risk of cancer: a population-based case-control study. Eur. J. Neurol. 21, 773–778 (2014).
Owe, J. F., Cvancarova, M., Romi, F. & Gilhus, N. E. Extrathymic malignancies in thymoma patients with and without myasthenia gravis. J. Neurol. Sci. 290, 66–69 (2010).
Norwood, F. et al. Myasthenia in pregnancy: best practice guidelines from a UK multispecialty working group. J. Neurol. Neurosurg. Psychiatry 85, 538–543 (2014). This article is a useful consensus document regarding treatment for MG for women of child-bearing potential and during pregnancy.
Gilhus, N. E. & Hong, Y. Maternal myasthenia gravis represents a risk for the child through autoantibody transfer, immunosuppressive therapy and genetic influence. Eur. J. Neurol. 25, 1402–1409 (2018).
Wolfe, G. I. et al. Myasthenia gravis activities of daily living profile. Neurology 52, 1487–1489 (1999).
Barnett, C., Bril, V., Kapral, M., Kulkarni, A. & Davis, A. M. Development and validation of the Myasthenia Gravis Impairment Index. Neurology 87, 879–886 (2016).
Burns, T. M., Grouse, C. K., Conaway, M. R. & Sanders, D. B. Construct and concurrent validation of the MG-QOL15 in the practice setting. Muscle Nerve 41, 219–226 (2010).
Burns, T. M. et al. The MG-QOL15 for following the health-related quality of life of patients with myasthenia gravis. Muscle Nerve 43, 14–18 (2011).
Burns, T. M. et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15R. Muscle Nerve 54, 1015–1022 (2016).
Padua, L. et al. Myasthenia gravis outcome measure: development and validation of a disease-specific self-administered questionnaire. Neurol. Sci. 23, 59–68 (2002).
Mullins, L. L., Carpentier, M. Y., Paul, R. H. & Sanders, D. B. Disease-specific measure of quality of life for myasthenia gravis. Muscle Nerve 38, 947–956 (2008).
Koopman, W. J., LeBlanc, N., Fowler, S., Hulley, D. & Nicolle, M. W. Hope, well-being, coping, and quality of life in adults with myasthenia gravis [abstract]. Muscle Nerve 52, S141 (2015).
Jaretzki, A. et al. Myasthenia gravis - recommendations for clinical research standards. Neurology 55, 16–23 (2000).
Burns, T. M., Conaway, M. & Sanders, D. B. The MG Composite: a valid and reliable outcome measure for myasthenia gravis. Neurology 74, 1434–1440 (2010). This article illustrates the importance of systematic testing and follow-up of patients with MG.
Behin, A. & Le Panse, R. New pathways and therapeutic targets in autoimmune myasthenia gravis. J. Neuromuscul. Dis. 5, 265–277 (2018). This article provides a nice overview of potential, new treatment targets in MG.
Mantegazza, R., Bernasconi, P. & Cavalcante, P. Myasthenia gravis: from autoantibodies to therapy. Curr. Opin. Neurol. 31, 517–525 (2018).
Molina, V. et al. Immunomodulation of experimental pulmonary fibrosis by intravenous immunoglobulin (IVIG). Autoimmunity 39, 711–717 (2006).
He, D. et al. Molecular and clinical relationship between live-attenuated Japanese encephalitis vaccination and childhood onset myasthenia gravis. Ann. Neurol. 84, 386–400 (2018). This article describes how specific vaccination may be involved in the onset of MG.
Makino, T. et al. Analysis of peripheral B cells and autoantibodies against the anti-nicotinic acetylcholine receptor derived from patients with myasthenia gravis using single-cell manipulation tools. PLOS ONE 12, e0185976 (2017).
Cron, M. A. et al. Analysis of microRNA expression in the thymus of myasthenia gravis patients opens new research avenues. Autoimmun. Rev. 17, 588–600 (2018).
Sabre, L., Maddison, P., Sadalage, G., Ambrose, P. A. & Punga, A. R. Circulating microRNA miR-21-5p, miR-150-5p and miR-30e-5p correlate with clinical status in late onset myasthenia gravis. J. Neuroimmunol. 321, 164–170 (2018).
Cruz, P. M. R., Huda, S., Lopez-Ruiz, P. & Vincent, A. Use of cell-based assays in myasthenia gravis and other antibody-mediated diseases. Exp. Neurol. 270, 66–71 (2015).
Trakas, N. & Tzartos, S. J. Immunostick ELISA for rapid and easy diagnosis of myasthenia gravis. J. Immunol. Methods 460, 107–112 (2018).
Valko, Y. et al. Ocular vestibular evoked myogenic potentials as a test for myasthenia gravis. Neurology 86, 660–668 (2016).
Chan, J. W. & Orrison, W. W. Ocular myasthenia: a rare presentation with MuSK antibody and bilateral extraocular muscle atrophy. Br. J. Ophthalmol. 91, 842–843 (2007).
Priola, A. M. et al. Comparison of CT and chemical-shift MRI for differentiating thymoma from non-thymomatous conditions in myasthenia gravis: value of qualitative and quantitative assessment. Clin. Radiol. 71, E157–E169 (2016).
Sala, T. P. et al. Efficacy and patient satisfaction in the use of subcutaneous immunoglobulin immunotherapy for the treatment of auto-immune neuromuscular diseases. Autoimmun. Rev. 17, 873–881 (2018).
Lazaridis, K., Dalianoudis, I., Baltatzidi, V. & Tzartos, S. J. Specific removal of autoantibodies by extracorporeal immunoadsorption ameliorates experimental autoimmune myasthenia gravis. J. Neuroimmunol. 312, 24–30 (2017).
Ulrichts, P. et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J. Clin. Invest. 128, 4372–4386 (2018). This article describes a class of drugs that may offer a new therapeutic approach in IgG-driven autoimmune disease.
Devanaboyina, S. C., Khare, P., Challa, D. K., Ober, R. J. & Ward, E. S. Engineered clearing agents for the selective depletion of antigen-specific antibodies. Nat. Commun. 8, 15314 (2017).
Lonze, B. E. et al. IdeS (Imlifidase): a novel agent that cleaves human IgG and permits successful kidney transplantation across high-strength donor-specific antibody. Ann. Surg. 268, 488–496 (2018).
Lipka, A. F. et al. Ephedrine treatment for autoimmune myasthenia gravis. Neuromuscul. Disord. 27, 259–265 (2017).
Sanders, D. B. et al. A double-blinded, randomized, placebo-controlled trial to evaluate efficacy, safety, and tolerability of single doses of tirasemtiv in patients with acetylcholine receptor-binding antibody-positive myasthenia gravis. Neurotherapeutics 12, 455–460 (2015).
Unwin, N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynapticmembranes. Q. Rev. Biophys. 46, 283–322 (2013).
Stiegler, A. L., Burden, S. J. & Hubbard, S. R. Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. J. Mol. Biol. 393, 1–9 (2009).
Zong, Y. N. et al. Structural basis of agrin-LRP4-MuSK signaling. Genes Dev. 26, 247–258 (2012).
Author information
Authors and Affiliations
Contributions
All authors have contributed to and are responsible for all parts of the Review. The first draft was written by N.E.G. (Introduction, Epidemiology and Overview of Primer); S.T. (Mechanisms/pathophysiology); J.P. (Diagnosis, screening and prevention); A.E. (Management); T.M.B. (Quality of life); and J.J.G.M.V. (Outlook).
Corresponding author
Ethics declarations
Competing interests
N.E.G. has received speaker’s honoraria from Octapharma and Alexion and consulting honoraria from Argenx and Ra Pharma. S.T. has shares in the research and diagnostic laboratory Tzartos NeuroDiagnostics. A.E. was a member of the advisory board for Alexion and is a scientific award jury member for Grifols and a safety data monitor for UCB. J.P. has received travel support or honoraria from MerckSerono, Biogen Idec, Novartis, Teva, Chugai Pharma, Bayer Schering, Alexion, Roche, Genzyme, Medimmune, Eurimmune, MedDay, Abide and Argenx and grants from MerckSerono, Novartis, Biogen Idec, Teva, Abide and Bayer Schering. T.M.B. was a member of the steering committee for Argenx. The institution of J.J.G.M.V. (Leiden University Medical Centre) has received fees from Alexion, Argenx and Ra Pharma owing to consultations by J.J.G.M.V. and has received royalties for antibody tests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gilhus, N.E., Tzartos, S., Evoli, A. et al. Myasthenia gravis. Nat Rev Dis Primers 5, 30 (2019). https://doi.org/10.1038/s41572-019-0079-y
Published:
DOI: https://doi.org/10.1038/s41572-019-0079-y
This article is cited by
-
Gut microbiota-derived butyrate restores impaired regulatory T cells in patients with AChR myasthenia gravis via mTOR-mediated autophagy
Cell Communication and Signaling (2024)
-
Integrative multi-omics analysis identifies genetically supported druggable targets and immune cell specificity for myasthenia gravis
Journal of Translational Medicine (2024)
-
An angel or a devil? Current view on the role of CD8+ T cells in the pathogenesis of myasthenia gravis
Journal of Translational Medicine (2024)
-
Distribution of multi-level B cell subsets in thymoma and thymoma-associated myasthenia gravis
Scientific Reports (2024)
-
Autoimmune diseases: targets, biology, and drug discovery
Acta Pharmacologica Sinica (2024)