Abstract
Fungicolous fungi are a very large, diverse, ecological and trophic group of organisms that are associated with other fungi. This association occurs with species of different lineages across the fungal kingdom. They are recognized as symbionts, mycoparasites, saprotrophs, and even neutrals. Wherever fungi have been found, fungicolous taxa have also been found. Homogeneous environments favour the development of highly adapted and coevolved fungicolous species, which could have led to host-specificity aspects. As a primary consumer, fungicolous fungi decrease the turnaround time of certain nutrients in food webs, due to their special often-rapid life cycles. They may also significantly affect population dynamics and population sizes of their hosts in aquatic or terrestrial ecosystems. As mycoparasites of pathogenic fungi, some fungicolous fungi have been explored as biocontrol agents. They may also cause serious diseases of cultivated edible and medicinal mushrooms, decreasing both yield and quality. Fungicolous fungi could be used as model organisms that may help determine better understanding of species interactions, fungal evolution and divergence, and fungicolous mechanisms. This review summarizes our current understanding of fungicolous fungi, with a particular focus on the terminology, diversity, global distribution, and interaction with their hosts. We also provide a checklist including 1552 fungicolous fungal taxa so far recorded following the updated classification schemes. There is a need for further investigations on this ecologically important group of fungi to better understand their biology, ecological aspects, origin and divergence, host-specificity and application in biocontrol. Accurate identification of these fungi as pathogens and their significance in quarantine purposes on the mushroom industry need further evaluations so that efficient control measures can be developed for better disease management purposes.




Similar content being viewed by others
Change history
26 April 2019
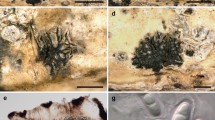
There were errors in Figs. 1 and 2 in the original publication. Figure panels 1a, 1b, 1h and 1p did not match the legend. The correct Fig. 1 is published below. In Fig. 2, the word Fusion should be Fusional.
References
Adams PB (1989) Comparison of antagonists of Sclerotinia species. Phytopathol 79(12):1345–1347
Aghayeva DN, Lutz M, Piątek M (2016) Transmission electron microscopy of Tuberculina species (Helicobasidiales) reveals a unique mode of conidiogenesis within Basidiomycota. Fungal Biol 120:1010–1016
Amasya AF, Narisawa K, Watanabe M (2015) Analysis of aclerotia-associated fungal communities in cool-temperate forest soils in north Japan. Microbes Environ 30:113–116
Arnolds E (1992) Macrofungal communities outside forests. In: Winterhoff W (ed) Fungi in vegetation science. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 113–150
Arnold JD (1935) A comparative study of certain species of Marasmius and Collybia in culture. Mycologia 27(4):388–417
Atanasova L, Le Crom S, Gruber S, Coulpier F, Seidl-Seiboth V, Kubicek CP, Druzhinina IS (2013) Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genom 14(1):121. https://doi.org/10.1186/1471-2164-14-12
Ayers W, Adams P (1981) Mycoparasitism of sclerotial fungi by Teratosperma oligocladum. Can J Microb 27:886–892
Baiswar P, Ngachan S, Chandra S (2014) Ramularia coleosporii, a hyperparasite on Coleosporium plumeriae in India. J Phytopathol 163:407–410
Bandoni RJ (1987) Taxonomic overview of the Tremellales. Stud Mycol 30:87–110
Bardin S, Huang H (2001) Research on biology and control of Sclerotinia diseases in Canada. Can J Plant Pathol 23(1):88–98
Bandoni RJ (1998) On some species of Mycogloea. Mycoscience 39(1):31–36
Barnett HL (1963) The nature of mycoparasitism by fungi. Ann Rev Microbiol 17:1–14
Barnett HL (1964) Mycoparasitism. Mycologia 56:1–19
Barnett HL, Binder FL (1973) The fungal host-parasite relationship. Annu Rev Phytopathol 11:273–292
Bartkowska A (2007) Parasitism of rust fungi spores by Ramularia species. Phytopatol Pol 43:61–67
Bauer R, Begerow D, Sampaio JP, Weiβ M, Oberwinkler F (2006) The simple-septate basidiomycetes: a synopsis. Mycol Prog 5(1):41–66
Bauer R, Lutz M, Oberwinkler F (2004) Tuberculina-rusts: a unique basidiomycetous interfungal cellular interaction with horizontal nuclear transfer. Mycologia 96:960–967
Bauer R, Oberwinkler F (1991) The colacosomes: new structures at the host-parasite interface of a mycoparasitic basidiomycete. Bot Acta 104:53–257
Bauer R, Oberwinkler F (2008) Cellular basidiomycete–fungus interactions. In: Varma A, Abbott L, Werner D, Hampp R (eds) Plant surface microbiology. Springer, Berlin, pp 267–279
Begerow D, Kemler M, Feige A, Yurkov A (2017) Parasitism in yeasts. In Buzzini P, Lachance MA, Yurkov AM (ed) Yeasts in natural ecosystems: ecology. Springer, Heidelberg, pp 179–210
Benjamin RK (1963) Addenda to “the merosporangiferous mucorales” II. Aliso J Syst Evol Botany 5:273–288
Benjamin RK (1979) Zygomycetes and their spores. In Kendrick WB (ed) The whole fungus, the sexual-asexual synthesis, vol 1. National Museum of Natural Sciences, Ottawa, Canada, pp 573–616
Benjamin RK (1985) A new genus of the Piptocephalidaceae (Zoopagales) from Japan. Bot J Linn Soc 91(1–2), 117–133
Benny GL, Benjamin RK (1976) Observations on Thamnidiaceae (Mucorales). II. Chaetocladium, Cokeromyces, Mycotypha, and Phascolomyces. Aliso: J Syst Evol Bot 8(4):391–424
Benny GL, Smith ME, Kirk PM, Tretter ED, White MM (2016) Challenges and future perspectives in the systematics of Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In: Li DW (ed) Biology of microfungi, fungal biology. Springer, Basel, pp 65–126
Benuzzi M, Baldoni G (2000) AQ 10, a new biofungicide based on Ampelomyces quisqualis for powdery mildew control on grapes. Inf Fitopatol 50:33–36
Berndt R (2013) Revision of the rust genus Uromyces on Cucurbitaceae. Mycologia 105:760–780
Beyer DM, O'Donnell K, Paley K, Wach MP (2013) First report of Syzygites megalocarpus (Mucorales) web mold on the commercial portabella button mushroom Agaricus bisporus in North America. Plant Dis 97(1):142
Binder FL, Barnett HL (1973) Enzymes for carbohydrate catabolism in the mycoparasite Tieghemiomyces parasiticus. Mycologia 65:999–1006
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98(6):971–981
Black JA (2012) The epidemiology of Puccinia emaculata (rust) in switchgrass and evaluation of the mycoparasite Sphaerellopsis filum as a potential biological control organism for switchgrass rust. Thesis, University of Tennessee, Knoxville
Blackwell M (2011) The fungi: 1, 2, 3 … 5.1 million species? Am J Bot 98:426–438
Boddy L (2000) Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol Ecol 31:185–194
Boddy L (2016) Interactions between fungi and other microbes. In: Watkinson SC, Boddy L, Money NP (eds) The fungi. Elsevier, London, pp 337–360
Boekhout T, Bandoni RJ, Fell JW, Kwon-Chung KJ (1998) Discussion of teleomorphic and anamorphic genera of the heterobasidiomycetous yeasts. In: Kurtzman CP, Fell JW (eds) The yeasts, a taxonomic study. Elsevier, Amsterdam, pp 609–625
Borges AV, Saraiva RM, Maffia LA (2015) Biocontrol of gray mold in tomato plants by Clonostachys rosea. Trop Plant Pathol 40:71–76
Boosalis MG (1964) Hyperparasitism. Annu Rev Phytopathol 2:363–376
Both EE (2006) Personal encounters with the parasitic bolete. Field Mycol 7:104–110
Braun U (1998) A monograph of Cercosporella, Ramularia, and allied genera (phytopathogenic hyphomycetes), vol 2. Ihw-Verlag, Eching
Braun U, Nakashima C, Crous PW (2013) Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, pteridophyta and gymnospermae. IMA Fungus 4:265–345
Burmester A, Karimi S, Wetzel J, Wöstemeyer J (2013) Complementation of a stable Met2-1 mutant of the zygomycete Absidia glauca by the corresponding wild-type allele of the mycoparasite Parasitella parasitica, transferred during infection. Microbiology 159:1639–1648
Byler JW, Cobb FW, Parmeter JR (1972) Occurrence and significance of fungi inhabiting galls caused by Peridermium harknessii. Can J Bot 50:1275–1282
Campos HD, Campos VP (1997) Effect of timing and forms of application of Arthrobotrys conoides, Arthrobotrys musiformis, Paecilomyces lilacinus and Verticillium chlamydosporium in the soil for the control of Meloidogyne exigua of coffee. Fitopatol Brasil 22:361–365
Canter HM, Ingold CT (1984) A chytrid on Dacrymyces. Trans Brit Mycol Soci 82:739–742
Carini P, Marsden PJ, Leff JW, Morgan EE, Strickland MS, Fierer N (2017) Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nature Microbiol 2:16242. https://doi.org/10.1038/nmicrobiol.2016.242
Castrillo LA, Hajek AE (2015) Detection of presumptive mycoparasites associated with Entomophaga maimaiga resting spores in forest soils. J Invertebr Pathol 124:87–89
Chalutz E, Wilson CL (1990) Postharvest biocontrol of green and blue mold andsour rot of Citrus-fruit by Debaryomyces hansenii. Plant Dis 74:134–137
Chaverri P, Samuels GJ (2013) Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 67:2823–2837
Chen CJ (1998) Morphological and molecular studies in the genus Tremella. Bibl Mycol 174:1–225
Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Persoh D, Dhami MK, Alias AS, Xu JC, Liu XZ, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Cooke RC (1977) The biology of symbiotic fungi. Wiley, New York
Cooke RC, Rayner AD (1984) Ecology of saprotrophic fungi. Longman, London, pp 1–415
Couch JN (1945) Observations on the genus Catenaria. Mycologia 37:163–193
Curtis F, Evans G, Lillis V, Lewis D, Cooke R (1978) Studies on mucorelean mycoparasites. New Phytol 80:157–165
Danell E (1999) Cantharellus. In: Cairney JWG, Chambers SM (eds) Ectomycorrhizal fungi key genera in profile. Springer-Verlag, Berlin, pp 253–267
Daniels BA, Menge JA (1980) Hyperparasitization of vesicular-arbuscular mycorrhizal fungi. Phytopathol 70(7):584–588
Dargan JS, Bhatia M (1986) A new variety of Hypomyces aurantius (Pers.: Fr.) Tul. from India. J Ind Bot Soc 65:53–55
De Beer ZW, Duong TA, Wingfield MJ (2016) The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol 83:165–191
De Hoog GS (1978) Notes some fungicolous hyphomycetes and their relatives. Persoonia 10:33–81
Deighton FC (1969) Microfungi. IV: some hyperparasitic hyphomycetes, and a note on Cercosporella uredinophila Sacc. Mycol Papers 118:1–41
Deighton FC, Pirozynski KA (1972) Microfungi V. More hyperparasitic hyphomycetes. Mycol Papers 128:1–110
Deyrup ST, Swenson DC, Gloer JB, Wicklow DT (2006) Caryophyllene sesquiterpenoids from a fungicolous isolate of Pestalotiopsis disseminata. J Nat Prod 69:608–611
Dogma IJ Jr, Sparrow FK (1969) A hyperparasitic Blyttiomyces. Mycologia 11:49–1158
Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E, Mukherjee PK, Zeilinger S, Grigoriev IV, Kubicek CP (2011) Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol 9:749–759
Ellenberger S, Burmester A, Wöstemeyer J (2014) Complete mitochondrial DNA sequence of the mucoralean fusion parasite Parasitella parasitica. Genome Announc 2(6):e00912-14. https://doi.org/10.1128/genomeA.00912-14
Ellenberger S, Burmester A, Wöstemeyer J (2016) Complete mitochondrial DNA sequence of the mucoralean fungus Absidia glauca, a model for studying host-parasite interactions. Genome Announc 4(2):e00153-16. https://doi.org/10.1128/genomeA.00153-16
Ellenberger S, Burmester A, Wostemeyer J (2018) The fate of mitochondria after infection of the Mucoralean fungus Absidia glauca by the fusion parasite Parasitella parasitica: comparison of mitochondrial genomes in zygomycetes. Mitochondrial DNA A DNA Mapp Seq Anal 29:113–120
Ellis MB, Ellis JP (1988) Microfungi on miscellaneous substrates: an identification handbook. The Richmond Publishing Co., Ltd, England, pp 1–244
Freeman K, Martin A, Karki D, Lynch R, Mitter M, Meyer A, Longcore J, Simmons D, Schmidt S (2009) Evidence that chytrids dominate fungal communities in high-elevation soils. Proc Natl Acad Sci 106:18315–18320
Friebes G (2012) A key to the non-lichenicolous species of the genus Capronia (Herpotrichiellaceae). Ascomycete Org 4:55–64
Fries EM, Nordholm J (1817) Symbolae Gasteromycorum ad illustrandam floram svecicam. Quas, Venia Ampl. Ord. Phil. In: Acad. Lund. Ex officina Berlingiana. Fasc, pp 1–25
Foulongne-Oriol M, Rodier A, Rousseau T, Largeteau M, Savoie J-M (2011) Quantitative genetics to dissect the fungal–fungal interaction between Lecanicillium verticillium and the white button mushroom Agaricus bisporus. Fungal Biol 115:421–431
Gafni A, Calderon CE, Harris R, Buxdorf K, Dafa-Berger A, Zeilinger-Reichert E, Levy M (2015) Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front Plant Sci 6:132. https://doi.org/10.3389/fpls.2015.00132
Gams W (1977) A key to the species of Mortierella. Persoonia 9:381–391
Gams W, Diederich P, Põldmaa K (2004) Fungicolous fungi. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring methods. Academic Press, Amsterdam, pp 343–392
Gea F, Pardo-Giménez A, Martínez-Carrasco A, Navarro M, Zied D (2010) Damage caused by false truffle (Diheliomyces microsporus) to Agaricus blazei mushroom crops in Spain. Bol Sanid Veg Plagas 36:233–238
Genne DM, De La Vega RCR, Branco S, Giraud T (2014) Fungal evolutionary genomics provides insight into the mechanisms of adaptive divergence in eukaryotes. Mol Ecol 23(4):753–773
Gilman JC, Tiffany LH (1952) Fungicolous fungi from Iowa. Proc Iowa Acad Sei 59:99–110
Gladieux P, Ropars J, Badouin H, Branca A, Aguileta G, De Vienne DM, Rodríguez de la Vega RC, Branco S, Giraud T (2014) Fungal evolutionary genomics provides insight into the mechanisms of adaptive divergence in eukaryotes. Mol Ecol 23(4):753–773
Ginns J (1986) The genus Syzygospora (Heterobasidiomycetes: Syzygosporaceae). Mycologia 78(4):619–636
Gleason FH, Carney LT, Lilje O, Glockling SL (2012) Ecological potentials of species of Rozella (Cryptomycota). Fungal Ecol 5:651–656
Gleason FH, Lilje O, Lange L (2017) What has happened to the “aquatic phycomycetes”(sensu Sparrow)? Part II: shared properties of zoosporic true fungi and fungus-like microorganisms. Fungal Biol Rev 32:52–61
Gleason FH, Lilje O, Marano AV, Sime-Ngando T, Sullivan BK, Kirchmair M, Neuhauser S (2014) Ecological functions of zoosporic hyperparasites. Front Microbiol 5:244. https://doi.org/10.3389/fmicb.2014.00244
Goh YK, Vujanovic V (2010) Sphaerodes quadrangularis biotrophic mycoparasitism on Fusarium avenaceum. Mycologia 102:757–762
Gray DJ, Morganjones G (1981) Host-parasite relationships of Agaricus brunnescens and a number of mycoparasitic hyphomycetes. Mycopathologia 75:55–59
Grishkan I, Zaady E, Nevo E (2006) Soil crust microfungi along a southward rainfall gradient in desert ecosystems. Eur J Soil Biol 42:33–42
Gu YH, Ko WH (1997) Water agarose medium for studying factors affecting germination of conidia of Ampelomyces quisqualis. Mycol Res 101:422–424
Gupta R, Mukerji K (2010) Mycoparasites in disease control. In: Mukerji KG, Manoharachary C (eds) Taxonomy and ecology of Indian fungi. IK International Pvt Ltd., pp 251–262
Hadar Y, Papadopoulou KK (2012) Suppressive composts: microbial ecology links between abiotic environments and healthy plants. Annu Rev Phytopathol 50:133–153. https://doi.org/10.1146/annurev-phyto-081211-172914
Hajek AE, Longcore JE, Simmons DR, Peters K, Humber RA (2013) Chytrid mycoparasitism of entomophthoralean azygospores. J Invertebr Pathol 114:333–336
Hargreaves J, Brickle P, Van West P (2018) The fungal ecology of seabird nesting sites in the Falkland Islands indicates a niche for mycoparasites. Fungal Ecol 36:99–108
Hashioka Y, Nakai Y (1980) Ultrastructure of pycnidial development and mycoparasitism of Ampelomyces quisqualis parasitic on Erysiphales. Trans Mycol Soc JP 21:329–338
Hatvani L, Antal Z, Manczinger L, Szekeres A, Druzhinina IS, Kubicek CP, Nagy A, Nagy E, Vagvolgyi C, Kredics L (2007) Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology 97:532–537
Hawksworth DL (1981) A survey of the fungicolous conidial fungi. In: Cole GT, Kendrick B (eds) Biology of conidial fungi. Academic Press, New York, pp 71–244
Hawksworth DL, Luecking R (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0052-2016
He P, He X, Zhang C (2006) Interactions between Psilocybe fasciata and its companion fungus Acremonium strictum. Ecol Res 21:387–395
Held AA (1981) Rozella and Rozellopsis: naked endoparasitic fungi which dress-up as their hosts. The Bot Rev 47(4):451–515
Helfer W (1991) Pilze auf Pilzfruchtkörpern. Untersuchungen zur Ökologie. Syst Chem Libri Bot 1:1–157
Hemmes DE, Desjardin DE (2001) A Burst of mycological activities in Hawaii in the 1990’s. Harvard Papers Bot 6:117–122
Herrera CS, Hirooka Y, Chaverri P (2016) Pseudocospeciation of the mycoparasite Cosmospora with their fungal hosts. Ecol Evol 6:1504–1514
Hoch H, Abawi G (1979) Mycoparasitism of oospores of Pythium ultimum by Fusarium merismoides. Mycologia 71(3):621–625
Hoffmann K, Pawłowska J, Walther G, Wrzosek M, De Hoog G, Benny G, Kirk P, Voigt K (2013) The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia 30:57–76
Hoffmann K, Voigt K (2009) Absidia parricida plays a dominant role in biotrophic fusion parasitism among mucoralean fungi (Zygomycetes): lentamyces, a new genus for A. parricida and A. zychae. Plant Biol 11:537–554
Hoffmann K, Voigt K, Kirk P (2011) Mortierellomycotina subphyl. nov., based on multi-gene genealogies. Mycotaxon 115:353–363
Holm L (1975) Taxonomic notes on ascomycetes. VIII. Microfungi on Cassiope tetragona. Svensk Bot Tidsk 69:143–160
Hoppe B, Purahong W, Wubet T, Kahl T, Bauhus J, Arnstadt T, Hofrichter M, Buscot F, Krüger D (2016) Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Divers 77:367–379
Howell C (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis 87:4–10
Hu XJ, Webster G, Xie LH, Yu CB, Li YS, Liao X (2013) A new mycoparasite, Aspergillus sp. ASP-4, parasitizes the sclerotia of Sclerotinia sclerotiorum. Crop Prot 54:15–22
Huang HC, Kokko EG (1993) Trichothecium roseum, a mycoparasite of Sclerotinia sclerotiorum. Can J Bot 71:1631–1638
Huang SK, Maharachchikumbura SSN, Jeewon R, Bhat JB, Phookamsak R, Hyde KD, Alsadi AM, Kang JC (2018) Lecanicillium subprimulinum (Cordycipitaceae, Hypocreales), a novel species from Baoshan, Yunnan. Phytotaxa 348:99–108
Hunter WE, Butler EE (1975) Syncephalis californica, a mycoparasite inducing giant hyphal swellings in species of Mucorales. Mycologia 67:863–872
Hyde KD, Maharachchikumbura SSN, Hongsanan S, Samarakoon MC, Lucking R, Pem D, Harishchandra D, Jeewon R, Zhao RL, Xu JC, Liu JK, Al-Sadi AM, Bahkali AH, Elgorban AM (2017) The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Divers 84:1–23
Inglis GD, Kawchuk LM (2002) Comparative degradation of oomycete, ascomycete, and basidiomycete cell walls by mycoparasitic and biocontrol fungi. Can J Microbiol 48:60–70
Jacques MA, Arlat M, Boulanger A, Boureau T, Carrère S, Cesbron S, Chen NW, Cociancich S, Darrasse A, Denancé N, Fischer-Le Saux M (2016) Using ecology, physiology, and genomics to understand host specificity in Xanthomonas. Ann Rev Phytopathol 54:163–187
Jacobs K, Holtzman K, Seifert KA (2005) Morphology, phylogeny and biology of Gliocephalis hyalina, a biotrophic contact mycoparasite of Fusarium species. Mycologia 97:111–120
Jager G, Velvis H (1985) Biological control of Rhizoctonia solani on potatoes by antagonists. 4. Inoculation of seed tubers with Verticillium biguttatum and other antagonists in field experiments. Eur J Plant Pathol 91:49–63
Jaklitsch WM, Voglmayr H (2015) Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud Mycol 80:1–87
James TY, Pelin A, Bonen L, Ahrendt S, Sain D, Corradi N, Stajich JE (2013) Shared signatures of parasitism and phylogenomics unite cryptomycota and microsporidia. Curr Biol 23:1548–1553
Jeffries P (1995) Biology and ecology of mycoparasitism. Can J Bot 73:S1284–S1290
Jeffries P, Kirk PM (1976) New technique for the isolation of mycoparasitic Mucorales. Trans Br Mycol Soc 66(3):541–543
Jeffries P, Young TWK (1976) Ultrastructure of infection of Cokeromyces recurvatus by Piptocephalis unispora (Mucorales). Arch Microbiol 109(3):277–288
Jiang XZ, Yu HY, Xiang MC, Liu XY, Liu XZ (2011) Echinochlamydosporium variabile, a new genus and species of Zygomycota from soil nematodes. Fungal Divers 46:43–51
Johnston PR (1999) Some hyperparasites of Meliolina in New Zealand. NZ J Bot 37:289–295
Jones MD, Richards TA, Hawksworth DL, Bass D (2011) Validation and justification of the phylum name Cryptomycota phyl. nov. IMA Fungus 2(2):173–175
Jordan EG, Barnett HL (1978) Nutrition and parasitism of Melanospora zamiae. Mycologia 7:300–312
Junker K, Ruiz GB, Lorenz A, Walker L, Gow NA, Wendland J (2018) The mycoparasitic yeast Saccharomycopsis schoenii predates and kills multi-drug resistant Candida auris. Sci Rep 8:14959. https://doi.org/10.1038/s41598-018-33199-z
Karlsson M, Atanasova L, Jensen DF, Zeilinger S (2017) Necrotrophic mycoparasites and their genomes. Spectrum. https://doi.org/10.1128/microbiolspec.FUNK-0016-2016
Karlsson M, Durling MB, Choi J, Kosawang C, Lackner G, Tzelepis GD, Nygren K, Dubey MK, Kamou N, Levasseur A (2015) Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. Genome Biol Evol 7:465–480
Karpov SA, Mikhailov KV, Mirzaeva GS, Mirabdullaev IM, Mamkaeva KA, Titova NN, Aleoshin VV (2013) Obligately phagotrophic aphelids turned out to branch with the earliest-diverging fungi. Protist 164:195–205
Katumoto K (1986) Two new species of Eudarluca hyperparasitic to Botryosphaeria. Trans Mycol Soc Jpn 27:11–16
Kellner M, Burmester A, Wöstemeyer A, Wöstemeyer J (1993) Transfer of genetic information from the mycoparasite Parasitella parasitica to its host Absidia glauca. Curr Genetics 23:334–337
Kepler RM, Sung GH, Harada Y, Tanaka K, Tanaka E, Hosoya T, Bischoff JF, Spatafora JW (2012) Host jumping onto close relatives and across kingdoms by Tyrannicordyceps (Clavicipitaceae) gen. nov. and Ustilaginoidea (Clavicipitaceae). Am J of Bot 99(3):552–561
Klecan AL, Hippe S, Somerville SC (1990) Reduced growth of Erysiphe graminis f. sp. hordei induced by Tilletiopsis pallescens. Phytopathology 80:325–331
Kim CS, Shirouzu T, Nakagiri A, Sotome K, Maekawa N (2013) Trichoderma eijii and T. pseudolacteum, two new species from Japan. Mycol Prog 12:739–753
Kim JJ, Goettel MS, Gillespie DR (2010) Evaluation of Lecanicillium longisporum, Vertalec® against the cotton aphid, Aphis gossypii, and cucumber powdery mildew, Sphaerotheca fuliginea in a greenhouse environment. Crop Prot 29:540–544
Kim M, Ahn C, Kim C (2017) The fungicolous ascomycetes genus Hypomyces in Korea. Mycobiol 45:209–212
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth & Bisby’s dictionary of the fungi. Cabi, UK
Kirschner R, Sampaio JP, Gadanho M, Weiß M, Oberwinkler F (2001) Cuniculitrema polymorpha (Tremellales, gen. nov. and sp. nov.), a heterobasidiomycete vectored by bark beetles, which is the teleomorph of Sterigmatosporidium polymorphuma. Anton Leeuw Int J G 80:149–161
Kiss L, Pintye A, Zséli G, Jankovics T, Szentiványi O, Hafez YM, Cook RT (2010) Microcyclic conidiogenesis in powdery mildews and its association with intracellular parasitism by Ampelomyces. Eur J Plant Pathol 126:445–451
Kosawang C, Karlsson M, Jensen DF, Dilokpimol A, Collinge DB (2014) Transcriptomic profiling to identify genes involved in Fusarium mycotoxin deoxynivalenol and zearalenone tolerance in the mycoparasitic fungus Clonostachys rosea. BMC Genom 15(1):55. https://doi.org/10.1186/1471-2164-15-55
Kouser S, Shah S (2013) Isolation and identification of Mycogone perniciosa, causing wet bubble disease in Agaricus bisporus cultivation in Kashmir. Afr J Agric Res 8:4804–4809
Krauss U, Ten Hoopen M, Rees R, Stirrup T, Argyle T, George A, Arroyo C, Corrales E, Casanoves F (2013) Mycoparasitism by Clonostachys byssicola and Clonostachys rosea on Trichoderma spp. from cocoa (Theobroma cacao) and implication for the design of mixed biocontrol agents. Biol Control 67:317–327
Krings M, Dotzler N, Longcore JE, Taylor TN (2010) An unusual microfungus in a fungal spore from the Lower Devonian Rhynie Chert. Palaeontology 53:753–759
Krings M, Dotzler N, Taylor TN (2009) Globicultrix nugax nov. gen. et nov. spec. (Chytridiomycota), an intrusive microfungus in fungal spores from the Rhynie chert. Zitteliana 48(49):165–170
Krings M, Dotzler N, Taylor TN (2011) Mycoparasitism in Dubiocarpon, a fungal sporocarp from the Carboniferous. Neues Jahrb Geol P-A 262:241–245
Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, Martinez DA, Druzhinina IS, Thon M, Zeilinger S, Casas-Flores S, Horwitz BA, Mukherjee PK (2011) Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol 12(4):R40. https://doi.org/10.1186/gb-2011-12-4-r40
Lachance MA, Pupovac-Velikonja A, Natarajan S, Schlag-Edler B (2000) Nutrition and phylogeny of predacious yeasts. Can J Microbiol 46:495–505
Lachance MA, Pang WM (1997) Predacious yeasts. Yeast 13:225–232
Lafferty KD, Allesina S, Arim M, Briggs CJ, De Leo G, Dobson AP, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546
Larena I, Torres R, De Cal A, Liñán M, Melgarejo P, Domenichini P, Bellini A, Mandrin JF, Lichou J, De Eribe XO, Usall J (2005) Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol Control 32(2):305–310
Lawrey JD, P Diederich (2018) Lichenicolous fungi—worldwide checklist, including isolated cultures and sequences available. http://www.lichenicolous.net. Accepted 1 Mar 2018
Lee S, Nam SH (2000) A mycoparasitic ascomycete Syspastospora parasitica on the entomopathogenic fungus Paecilomyces tenuipes growing in Bombyx mori. Mycobiology 28:130–132
Leonardi M, Ascione S, Pacioni G, Cesare P, Pacioni ML, Miranda M, Zarivi O (2018) The challenge for identifying the fungi living inside mushrooms: the case of truffle inhabiting mycelia. Plant Biosyst 152:1002–1010
Letcher PM, Powell MJ (2002) A taxonomic summary of chytriomyces (Chytridiomycota). Mycotaxon 84:447–488
Letcher PM, Longcore JE, Quandt CA, da Silva Leite D, James TY, Powell MJ (2017) Morphological, molecular, and ultrastructural characterization of Rozella rhizoclosmatii, a new species in cryptomycota. Fungal Biol 121:1–10
Li DC, Shen CY (1996) Olpitrichum tenellum as a biotrophic contact mycoparasite. Can J Bot 74:2014–2016
Li Y, Hyde KD, Jeewon R, Cai L, Vijaykrishna D, Zhang K (2005) Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear & protein coding genes. Mycologia 97:1034–1046
Liu AR, Chen SC, Wu SY, Xu T, Guo LD, Jeewon R, Wei JG (2010) Cultural studies coupled with DNA based sequence analyses and its implication on pigmentation as a phylogenetic marker in Pestalotiopsis taxonomy. Mol Phylogenetics Evol 57:528–535
Liu P, Yoshimichi D, Wang X, Wang Q (1999) The Hypocreaceae of China III. Some fungicolous species of the genus Hypocrea. Mycosystema 19(3):317–327
Liu XZ, Wang QM, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT, Millanes AM, Wedin M, Yurkov AM, Boekhout T, Bai FY (2015) Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 81:85–147
Lorito M, Peterbauer TC, Hayes CK, Harman GE (1994) Synergistic interaction between fungal cell-wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiol-UK 140:623–629
Madi L, Katan T, Katan J, Henis Y (1997) Biological control of Sclerotium rolfsii and Verticillium dahliae by Talaromyces flavus is mediated by different mechanisms. Phytopathology 87:1054–1060
Malloch D, Mallik A (1998) Taxonomy of Orphnodactylis kalmiae gen. et sp. nov. influenced by the hyperparasite Didymosphaeria kalmiae. Can J Bot 6(7):1265–1275
Marano AV, Pires-Zottarelli CLA, Barrera MD, Steciow MM, Gleason FH (2011) Diversity, role in decomposition, and succession of zoosporic fungi and straminipiles on submerged decaying leaves in a woodland stream. Hydrobiologia 659:93–109
McKenzie E, Johnston P, Buchanan P (2006) Checklist of fungi on teatree (Kunzea and Leptospermum species) in New Zealand. N Zeal J Bot 4:293–335
Melo IS, Faull JL, Nascimento RS (2006) Antagonism of Aspergillus terreus to Sclerotinia sclerotiorum. Braz J Microbiol 37(4):417–499
Melo RF, Maia LC, Santiago AL (2016) The discovery of Syncephalis obliqua (Zoopagomycotina, Zoopagales) in the neotropics. Mycotaxon 130:1165–1169
Melo RFR, de Luiz AS, Cavalcanti MAdQ (2011) Syncephalis clavata (Zoopagales, Zygomycetes), a first record from the neotropics. Mycotaxon 116:133–136
Mihál I, Cicák A, Bucinova K (2007) Distribution, ecology and taxonomical notes to species of genera Hypomyces and Nectria (Hypocreales) in Slovakia. Mikol I Fitopatol 41:242–251
Miles PG, Chang ST (1997) Mushroom biology: concise basics and current developments. World Scientific, Singapore
Millanes AM, Truong C, Westberg M, Diederich P, Wedin M (2014) Host switching promotes diversity in host-specialized mycoparasitic fungi: uncoupled evolution in the biatoropsis-ssnea system. Evolution 68:1576–1593
Millanes AM, Westberg M, Wedin M, Diederich P (2012) Tremella diploschistina (Tremellales, Basidiomycota, Fungi), a new lichenicolous species growing on Diploschistes. The Lichenol 44(3):321–332
Miyazaki K, Tsuchiya Y, Okuda T (2009) Specific PCR assays for the detection of Trichoderma harzianum causing green mold disease during mushroom cultivation. Mycoscience 50:94–99
Misra PC, Lata K (1979) Studies on dimargaritaceae (Mucorales). II. A new Dispira parasitic on ascomycetous hosts. Mycotaxon 8:372–376
Moore D, Robson GD, Trinci AP (2011) 21st century guidebook to fungi with CD. Cambridge University Press, Cambridge
Mudur SV, Gloer JB, Wicklow DT (2006) Sporminarins A and B: antifungal metabolites from a fungicolous isolate of Sporormiella minimoides. J Antibiot 59:500–506
Mueller GM, Schmit JP, Leacock PR, Buyck B, Cifuentes J, Desjardin DE, Halling RE, Hjortstam K, Iturriaga T, Larsson K-H (2007) Global diversity and distribution of macrofungi. Biodivers Conserv 16:37–48
Mukherjee PK, Horwitz BA, Herrera-Estrella A, Schmoll M, Kenerley CM (2013) Trichoderma research in the genome era. Annu Rev Phytopathol 51:105–129
Müller E, Petrini O, Fisher PJ, Samuels GJ, Rossman AY (1987) Taxonomy and anamorphs of the Herpotrichiellaceae with notes on generic synonymy. Trans Brit Mycol Soc 88:63–74
Nicoletti R, De Stefano M (2012) Penicillium restrictum as an antagonist of plant pathogenic fungi. Dyn Biochem Proc Biotech Mol Biol 6:61–69
Nicot J (1967) Clépur la détermination des espèces banales de champignons fongicoles. Rev Mycol 31:393–399
Niu XM, Zhang KQ (2011) Arthrobotrys oligospora: a model organism for understanding the interaction between fungi and nematodes. Mycology 2:59–78
Oberwinkler F (2012) Evolutionary trends in Basidiomycota. Stapefia 96:45–104
Oberwinkler F (2017) Yeasts in pucciniomycotina. Mycol Prog 16:831–856
Olsson S, Persson Y (1994) Transfer of phosphorus from Rhizoctonia solani to the mycoparasite Arthrobotrys oligospora. Mycol Res 98:1065–1068
Opik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier U, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241
Overton BE, Stewart EL, Geiser DM, Jaklitsch WM (2006) Systematics of Hypocrea citrina and related taxa. Stud Mycol 56:1–38
Pacioni G, Leonardi M (2016) Truffle-inhabitingfFungi. In: Zambonelli A, Iotti M, Murat C (Eds) True Truffle (Tuber spp.) in the world: soil ecology, systematics and biochemistry. Springer International Publishing, Cham (2016), pp 283–299
Pacioni G, Leonardi M, Aimola P, Ragnelli AM, Rubini A, Paolocci F (2007) Isolation and characterization of some mycelia inhabiting Tuber ascomata. Mycol Res 111:1450–1460
Parkinson D, Williams S (1960) A method for isolating fungi from soil microhabitats. Plant Soil 13:347–355
Parratt SR, Barrès B, Penczykowski RM, Laine AL (2017) Local adaptation at higher trophic levels: contrasting hyperparasite–pathogen infection dynamics in the field and laboratory. Mol Ecol 26(7):1964–1979
Parratt SR, Laine AL (2018) Pathogen dynamics under both bottom‐up host resistance and top‐down hyperparasite attack. J Appl Ecol 55(6):2976–2985
Pei MH, Yuan ZW (2005) Sphaerellopsis filum and its potential for biological control of willow rusts. In: Pei MH, McCracken AR (eds) Rust diseases of willow and poplar. CABI Publishing, Wallingford, pp 243–253
Peintner U, Schwarz S, Mešić A, Moreau PA, Moreno G, Saviuc P (2013) Mycophilic or mycophobic? Legislation and guidelines on wild mushroom commerce reveal different consumption behaviour in European countries. PloS ONE 8:e63926
Pennycook S (2009) The correct authorship of the genus Hypomyces and its original species. Mycotaxon 108:185–195
Persson Y, Friman E (1993) Intracellular proteolytic activity in mycelia of Arthrobotrys oligospora bearing mycoparasitic or nematode trapping structures. Exp Mycol 17:182–190
Perrott PE (1960) The ecology of some aquatic Phycomycetes. Trans Brit Mycol Soc 43:19–30
Piątek M (2002) Naohidea sebacea (Fungi, Urediniomycetes) in Poland: rediscovered after a century on a new host. Pol J Bot J 47:49–51
Pintye A, Bereczky Z, Kovacs GM, Nagy LG, Xu X, Legler SE, Vaczy Z, Vaczy KZ, Caffi T, Rossi V, Kiss L (2012) No indication of strict host associations in a widespread mycoparasite: grapevine powdery mildew (Erysiphe necator) is attacked by phylogenetically distant Ampelomyces strains in the field. Phytopathology 102:707–716
Pintye A, Ropars J, Harvey N, Shin HD, Leyronas C, Nicot PC, Giraud T, Kiss L (2015) Host phenology and geography as drivers of differentiation in generalist fungal mycoparasites. Plos ONE 10(3):e0120703. https://doi.org/10.1371/journal.pone.0120703
Pirozynski KA (1977) Notes on hyperparasitic sphaeriales, hypocreales and ‘hypocreoid dothideales’. Kew Bull 31:595–610
Poinar GO, Buckley R (2007) Evidence of mycoparasitism and hypermycoparasitism in early cretaceous amber. Mycol Res 111:503–506
Põldmaa K, Samuels GJ (1999) Aphyllophoricolous species of Hypomyces with KOH-negative perithecia. Mycologia 91:177–199
Põldmaa K (2000) Generic delimitation of the fungicolous hypocreaceae. Stud Mycol 45:83–94
Põldmaa K (2011) Tropical species of Cladobotryum and Hypomyces producing red pigments. Stud Mycol 68:1–34
Powell MJ (1981) Zoospore structure of the mycoparasitic chytrid Caulochytrium protostelioides Olive. Am J Bot 68:1074–1089
Powell MJ (1993) Looking at mycology with a Janus face: a glimpse at chytridiomycetes active in the environment. Mycologia 85:1–20
Powell MJ, Letcher PM, James TY (2017) Ultrastructural characterization of the host-parasite interface between Allomyces anomalus (Blastocladiomycota) and Rozella allomycis (Cryptomycota). Fungal Biol 121:561–572
Prillinger H, Lopandic K, Sugita T, Wuczkowski M (2007) Asterotremella gen. nov. albida, an anamorphic tremelloid yeast isolated from the agarics Asterophora lycoperdoides and Asterophora parasitica. J Gen Appl Microbiol 53:167–175
Quandt CA, Di Y, Elser J, Jaiswal P, Spatafora JW (2016) Differential expression of genes involved in host recognition, attachment, and degradation in the mycoparasite Tolypocladium ophioglossoides. G3 Genes Genomes Genetics 6:731–741
Quandt CA, Kepler RM, Gams W, Araújo JP, Ban S, Evans HC, Hughes D, Humber R, Hywel-Jones N, Li Z, Luangsa-Ard JJ (2014) Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 5(1):121–134
Quandt CA, Patterson W, Spatafora JW (2018) Harnessing the power of phylogenomics to disentangle the directionality and signatures of interkingdom host jumping in the parasitic fungal genus Tolypocladium. Mycologia 110:104–117
Ranković B (1997) Hyperparasites of the genus shape Ampelomyces on powdery mildew fungi in Serbia. Mycopathology 139:157–164
Reátegui RF, Wicklow DT, Gloer JB (2006) Phaeofurans and sorbicillin analogues from a fungicolous Phaeoacremonium species (NRRL 32148). J Nat Prod 69:113–117
Redhead SA, Ammirati JF, Walker GR, Norvell LL, Puccio MB (1994) Squamanita contortipes, the Rosetta Stone of a mycoparasitic agaric genus. Can J Bot 72(12):1812–1824
Redhead SA, Seifert KA (2001) Asterophora Ditmar ex Link 1809 versus Nyctalis Fries 1825, and the status of Ugola Adanson 1763. Taxon 50(1):243–268
Reid DA (1990) New or interesting records of British Heterobasidiomycetes. Mycol Res 94(1):94–108
Reynolds NK, Benny GL, Ho HM, Hou YH, Crous PW, Smith ME (2019) Phylogenetic and morphological analyses of the mycoparasitic genus Piptocephalis. Mycologia. https://doi.org/10.1080/00275514.2018.1538439
Richardson MJ (2002) The coprophilous succession. Fungal Divers 10:1–111
Rogerson CT, Samuels GJ (1989) Boleticolous species of Hypomyces. Mycologia 81:413–432
Rogerson CT, Samuels GJ (1993) Polyporicolous species of Hypomyces. Mycologia 85:231–272
Rogerson CT, Samuels GJ (1994) Agaricicolous species of Hypomyces. Mycologia 86:839–866
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological-control agents—theory and evidence. Biol Control 5:303–335
Rossman AY (1987) The Tubeufiaceae and similar loculoascomycetes. Mycol Paper 157:1–71
Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999) Genera of bionectriaceae, hypocreaceae and nectriaceae (Hypocreales, Ascomycetes). Stud Mycol 42:1–248
Rossman AY, Seifert KA, Samuels GJ, Minnis AM, Schroers H-J, Lombard L, Crous PW, Põldmaa K, Cannon PF, Summerbell RC (2013) Genera in bionectriaceae, hypocreaceae, and nectriaceae (hypocreales) proposed for acceptance or rejection. IMA Fungus 4:41–51
Potocnik I, Vukojevic J, Stajic M, Rekanovic E, Milijasevic S, Todorovic B, Stepanovic M (2009) In vitro toxicity of selected fungicides from the groups of benzimidazoles and demethylation inhibitors to Cladobotryum dendroides and Agaricus bisporus. J Environ Sci Heal B 44:365–370
Rudakov OL (1978) Physiological groups in mycophilic fungi. Mycologia 70:150–159
Rudakov OL (1981) Kikofil’nye Griby Ikh Biologiya i. Prakticheskoe Znachenie, Moscow, pp 1–160
Samuels GJ (1988) Fungicolous, lichenicolous, and myxomyceticolous species of Hypocreopsis, Nectriopsis, Nectria, Peristomialis, and Trichonectria. Mem N Y Bot Gard 48:1–78
Samuels GJ, Candoussau F, Magni JF (1997) Fungicolous pyrenomycetes 2. Ascocodinaea, gen nov., and reconsideration of litschaueria. Mycologia 89:156–162
Samuels GJ, Seifert KA (1987) Taxonomic implications of variation among hypocrealean anamorphs. In: Sugiyama J (ed) Pleomorphic Fungi. The Diversity and Its Taxonomic Implications. Kodansha, Tokyo, pp 29–56
Schmoll M, Dattenbock C, Carreras-Villasenor N, Mendoza-Mendoza A, Tisch D, Aleman MI, Baker SE, Brown C, Cervantes-Badillo MG, Cetz-Chel JGR, Cristobal-Mondragon GR, Delaye L, Esquivel-Naranjo EU, Frischmann A, Gallardo-Negrete Jde J, Garcia-Esquivel M, Gomez-Rodriguez EY, Greenwood DR, Hernandez-Onate M, Kruszewska JS, Lawry R, Mora-Montes HM, Munoz-Centeno T, Nieto-Jacobo MF, Nogueira Lopez G, Olmedo-Monfil V, Osorio-Concepcion S, Pilsyk KR, Pomraning A, Rodriguez-Iglesias MT, Rosales-Saavedra M, Sanchez-Arreguin JA, Seidl-Seiboth V, Stewart A, Uresti-Rivera EE, Wang CL, Wang TF, Zeilinger S, Casas-Flores S, Herrera-Estrella A (2016) The genomes of three uneven siblings: footprints of the lifestyles of three Trichoderma species. Microbiol Mol Biol Rev 80:205–327
Seifert KA (1985) A monograph of Stilbella and some allied hyphomycetes. Stud Mycol 27:1–235
Sempere F, Santamarina M (2008) Suppression of Nigrospora oryzae (Berk. & Broome) petch by an aggressive mycoparasite and competitor, Penicillium oxalicum Currie & Thom. Int J Food Microbio 122:35–43
Shin TS, Yu NH, Lee J, Choi GJ, Kim J-C, Shin CS (2017) Development of a biofungicide using a mycoparasitic fungus Simplicillium lamellicola BCP and its control efficacy against gray mold diseases of tomato and ginseng. Plant Pathol J 33:337–344
Singh UB, Sahu A, Singh RK, Singh DP, Meena KK, Srivastava JS, Manna MC (2012) Evaluation of biocontrol potential of Arthrobotrys oligospora against Meloidogyne graminicola and Rhizoctonia solani in Rice (Oryza sativa L.). Biol Cont 60:262–270
Siozios S, Tosi L, Ferrarini A, Ferrari A, Tononi P, Bellin D, Maurhofer M, Gessler C, Delledonne M, Pertot I (2015) Transcriptional reprogramming of the mycoparasitic fungus Ampelomyces quisqualis during the powdery mildew host-induced germination. Phytopathology 105:199–209
Spatafora JW, Aime MC, Grigoriev IV, Martin F, Stajich JE, Blackwell M (2017) The fungal tree of life: from molecular systematics to genome-scale phylogenies. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0053-2016
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108(5):1028–1046
Srinivasan MC, Thirumalachar M (1965) Studies on species of Conidiobolus from India. Sydowia 19:86–91
Summerbell RC, Gueidan C, Schroers HJ, De Hoog GS, Starink M, Rosete YA, Guarro J, Scott JA (2011) Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud Mycol 68:139–162
Sun JZ, Dong CH, Liu XZ, Liu JK, Hyde KD (2016a) Calcarisporium cordycipiticola sp. nov., an important fungal pathogen of Cordyceps militaris. Phytotaxa 268:135–144
Sun JZ, Liu XZ, Hyde KD, Zhao Q, Maharachchikumbura SSN, Camporesi E, Bhat J, Nilthong S, Lumyong S (2017) Calcarisporium xylariicola sp. nov. and introduction of Calcarisporiaceae fam. nov. in Hypocreales. Mycol Prog 16:433–445
Sun JZ, Pei YF, Li EW, Li W, Hyde KD, Yin WB, Liu XZ (2016b) A new species of Trichoderma hypoxylon harbours abundant secondary metabolites. Sci Rep. https://doi.org/10.1038/srep37369
Sundheim L (1982) Control of cucumber powdery mildew by the hyperparasite Ampelomyces quisqualis and fungicides. Plant Pathol 31:209–214
Sung GH, Hywel-Jones NL, Sung JM, Luangsa-Ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Swe A, Jeewon R, Pointing SB, Hyde KD (2008a) Taxonomy and phylogeny of Arthrobotrys mangrovispora, a new marine nematode-trapping fungal species. Bot Mar 51(4):331–338
Swe A, Jeewon R, Hyde KD (2008b) Nematode-Trapping fungi from mangrove habitats. Cryptogam Mycol 29:333–354
Swe A, Li J, Zhang KQ, Pointing SB, Jeewon R, Hyde KD (2011) Nematode-trapping fungi. CurRes Environ Appl Mycol 1:1–26
Tamm H, Põldmaa K (2013) Diversity, host associations, and phylogeography of temperate aurofusarin-producing Hypomyces/Cladobotryum including causal agents of cobweb disease of cultivated mushrooms. Fungal Biol 117:348–367
Tanabe Y, O’Donnell K, Saikawa M, Sugiyama J (2000) Molecular phylogeny of parasitic Zygomycota (Dimargaritales, Zoopagales) based on nuclear small subunit ribosomal DNA sequences. Mol Phylogen Evol 16:253–262
Taylor JW, Berbee ML (2006) Dating divergences in the Fungal Tree of Life: review and new analyses. Mycologia 98(6):838–849
Tedersoo L, Sánchez-Ramírez S, Kõljalg U, Bahram M, Döring M, Schigel D, May T, Ryberg M, Abarenkov K (2018) High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers 90:135–159
Torres DE, Rojas-Martínez RI, Zavaleta-Mejía E, Guevara-Fefer P, Márquez-Guzmán GJ, Pérez-Martínez C (2017) Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PloS ONE 12(1): e0170782. https://doi.org/10.1371/journal.pone.0170782
Tubaki K (1955) Studies on the Japanese hyphomycetes. II. Fungicolous group. Nag Mycol J Nag Inst 5:11–40
Tyson JL, Henderson RC, Fullerton RA, Jamieson LE, Froud KJ (2005) Distribution and new host records for Cosmospora aurantiicola and Cosmospora flammea: entomopathogens of Diaspididae in New Zealand. NZ Plant Pro 58:283–287
Uma N, Taylor G (1987) Parasitism of leek rust urediniospores by four fungi. Trans Brit Mycol Soc 88:335–340
Upadhyaya RC, Sohi HS, Vijay B (1987) Cladobotryum apiculatum a new mycoparasite of Pleurotus beds. Indian Phytopath 40:294
Vandermeer J, Perfecto I, Liere H (2009) Evidence for hyperparasitism of coffee rust (Hemileia vastatrix) by the entomogenous fungus, Lecanicillium lecanii, through a complex ecological web. Plant Pathol 58:636–641
Videira SIR, Groenewald JZ, Verkley GJM, Braun U, Crous PW (2015) The rise of Ramularia from the Qrella labyrinth. Fungal Biol 119:823–843
Von Esenbeck CGN (1817) Das system der pilze und schwämme, vol 2. Stahelfchen, Berlin
Wakefield EM, Bisby GR (1941) List of hyphomycetes recorded for Britain. Trans Br Mycol Soc 25:49–126
Wang QM, Yurkov AM, Göker M, Lumbsch HT, Leavitt SD, Groenewald M, Theelen B, Liu XZ, Boekhout T, Bai FY (2015) Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Stud Mycol 1:149–189
Ward N, Robertson C, Chanda A, Schneider R (2012) Effects of Simplicillium lanosoniveum on Phakopsora pachyrhizi, the soybean rust pathogen, and its use as a biological control agent. Phytopathology 102:749–760
Ward N, Schneider R, Aime M (2011) Colonization of soybean rust sori by Simplicillium lanosoniveum. Fungal Ecol 4:303–308
Wei DP, Wanasinghe DN, Chaiwat TA, Hyde KD (2018) Lecanicillium uredinophilium known from rusts, also occurs animal hosts with chitinous bodies. Asian J Mocol 1:63–73
Whipps JM (1997) Interactions between fungi and plant pathogens in soil and the rhizosphere. In: Gange AC, Brown VK (eds) Multitrophic interactions in terrestrial systems. Blackwell Science, Oxford, pp 47–65
Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lucking R, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch HT, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castaneda-Ruiz RF, Bahkali AH, Pang KL, Tanaka K, Dai DQ, Sakayaroj J, Hujslova M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Caceres MES, Perez-Ortega S, Fiuza PO, Monteiro JS, Vasilyeva LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel-Wahab MA, Takamatsu S, Bensch K, de Silva NI, De Kesel A, Karunarathna A, Boonmee S, Pfister DH, Lu YZ, Luo ZL, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng XY, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera RH, Tang LZ, Phukhamsakda C, Hernandez-Restrepo M, Ma XY, Tibpromma S, Gusmao LFP, Weerahewa D, Karunarathna SC (2017a) Notes for genera: ascomycota. Fungal Divers 86:1–594
Wijayawardene NN, Hyde KD, Tibpromma S, Wanasinghe DN, Thambugala KM, Tian Q, Wang Y (2017b) Towards incorporating asexual fungi in a natural classification: checklist and notes 2012–2016. Mycosphere 8:1457–1555
Wijayawardene NN, Hyde KD, Lumbsch HT, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018a) Outline of ascomycota 2017. Fungal Divers 88:167–263
Wijayawardene NN, Pawłowska J, Letcher PM, Kirk PM, Humber RA, Schüßler A, Wrzosek M, Muszewska A, Okrasinska A, Istel L, Gesiorska A, Mungai P, Lateef AA, Rajeshkumar KC, Singh RV, Radek R, Walther G, Wagner L, Walker C, Wijesundara DSA, Papizadeh M, Dolatabadi S, Shenoy BD, Tokarev YS, Lumyong S, Hyde KD (2018b) Notes for genera: basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers 92:43–129
Willdenow CL, von Linné C, Link HF, Dietrich A, Schwägrichen CF, von Linné C, Link HF (1833) Caroli a Linné Species plantarum exhibentes plantas rite cognitas ad genera relatas cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas olim curante Carolo Ludovico Willdenow. Impensis GC Nauck, Berolini
Willoughby LG (1956) Studies on soil chytrids. I. Rhizidium richmondense sp. nov. and its parasites. Trans Br Mycol Soc 39:125–141
Wöstemeyer J, Ellenberger S, Schulz E, Siegmund L, von Burgeler A, Gerlitz N, Burmester A, Wetzel J, Voigt J (2016) Fusion parasitism between Parasitella parasitica and its host Absidia glauca: a system between sexuality and parasitism. Endocytobiosis Cell Res 27:24–32
Wöstemeyer J, Wöstemeyer A, Burmester A, Czempinski K (1995) Relationships between sexual processes and parasitic interactions in the host–pathogen system Absidia glauca–Parasitella parasitica. Can J Bot 73:243–250
Yang J, Yu Y, Li J, Zhu W, Geng ZY, Jiang DW, Wang YC, Zhang KQ (2013) Characterization and functional analyses of the chitinase-encoding genes in the nematode-trapping fungus Arthrobotrys oligospora. Arch Microbiol 195:453–462
Yuan ZW, Pei MH, Hunter T, Ruiz C, Royle DJ (1999) Pathogenicity to willow rust, Melampsora epitea, of the mycoparasite Sphaerellopsis filum from different sources. Mycol Res 103:509–512
Yurkov A, Kruger D, Begerow D, Arnold N, Tarkka MT (2012) Basidiomycetous yeasts from Boletales fruiting bodies and their interactions with the mycoparasite Sepedonium chrysospermum and the host fungus Paxillus. Microb Ecol 63:295–303
Zare R, Gams W (2008) A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol Res 112:811–824
Zare R, Gams W, Evans H (2001) A revision of Verticillium section Prostrata. V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwig 73:51–86
Zeilinger S, Omann M (2007) Trichoderma biocontrol: signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul Syst Biol 1:227–234
Zhang WW, Zhang XL, Li K, Wang CH, Cai L, Zhuang WY, Xiang MC, Liu X (2018) Introgression and gene family contraction drive the evolution of lifestyle and host shifts of hypocrealean fungi. Mycology 9:176–188
Zhao RL, Li GJ, Sanchez-Ramirez S, Stata M, Yang ZL, Wu G, Dai YC, He SH, Cui BK, Zhou JL, Wu F, He MQ, Moncalvo JM, Hyde KD (2017) A six-gene phylogenetic overview of basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Divers 84:43–74
Zhu ZX, Zhuang WY (2013) Resources of nonlichenized fungicolous Ascomycota from China. Mycosystema 32(suppl):79–88
Zugmaier W, Oberwinkler F (1995) Tremelloid haustorial cells with haustorial filaments and potential host range of Tremella mesenterica. Nord J Bot 15:207–213
Acknowledgments
This research was supported by the Natural Science Foundation of China (no. 31600024).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, JZ., Liu, XZ., McKenzie, E.H.C. et al. Fungicolous fungi: terminology, diversity, distribution, evolution, and species checklist. Fungal Diversity 95, 337–430 (2019). https://doi.org/10.1007/s13225-019-00422-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-019-00422-9