Abstract
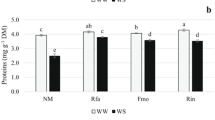
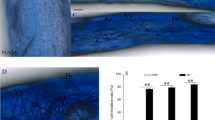
This experiment was carried out in pots in a greenhouse to evaluate the effects of arbuscular mycorrhizal fungi (Funneliformis mosseae, Rhizophagus intraradices and Rhizophagus fasciculatus) on carob plant performance under different levels of phosphate fertilization. Non-mycorrhizal (NMyc) and mycorrhizal (Myc) carob plants were subjected to three levels of phosphate fertilization, L1 (0 mg P kg−1 soil), L2 (25 mg P kg−1 soil) and L3 (100 mg P kg−1 soil). Results showed that under L1 and L2 P-fertilization levels, arbuscular mycorrhizal symbiosis significantly improved growth and biomass production of carob plants. Moreover, mineral nutrient (P, K, Na and Ca) acquisition, photosynthetic activity (Fv/Fm), stomatal conductance, total chlorophyll content, and soluble sugar accumulation were also strongly improved in Myc plants in comparison with NMyc ones. Under L1 P-fertilization level, Myc plants showed strongly increased acid phosphatase activity in roots and in the rhizospheric soil than NMyc plants. Furthermore, Myc plants maintained high membrane integrity (over 80%) and low hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents, associated with increased activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (G-POD), and catalase (CAT) compared to NMyc plants. However, high phosphorus input (L3) negatively affected root colonization and mycorrhizal plant performance. Thus, carob plants associated with Funneliformis mosseae performed best under phosphorus deficiency and were the least sensitive to the variations of phosphorus input levels.



Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Amako K, Chen GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Arafat AHA, Chaoxing H (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Hortic 127:228–233
Arnon DI (1949) Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris L. Plant Physiol 24:1–15
Bagayoko M, George E, Römheld V, Buerkert A (2000) Effects of mycorrhizae and phosphorus on growth and nutrient uptake of millet, cowpea and sorghum on a West African soil. J Agric Sci 135:399–407
Balzergue C, Chabaud M, Barker DG, Bécard G, Rochange SF (2013) High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spikin gresponses to the fungus. Front Plant Sci 4:426
Bargaz A, Faghire M, Farissi M, Drevon JJ, Ghoulam C (2013) Oxidative stress in the root nodules of Phaseolus vulgaris is induced under conditions of phosphorus deficiency. Acta Physiol Plant 35:1633–1644
Batlle I, Tous J (1997) Carob tree Ceratonia siliqua L. International Plant Genetic Resources Institute, Rome
Beltrano J, Ruscitti M, Arango MC, Ronco M (2013) Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and P levels. J Soil Sci Plant Nutr 13:123–141
Besford RT (1978) Effect of phosphorus supply on acid phosphatase activity in the leaves of tomato plants. Scient Hort 9:303–309
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bor M, Ozdemir F, Turkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84
Boukcim H, Mousain D (2001) Effets de la fertilisation phosphatée sur la mycorhization, la croissance et la nutrition en phosphore et en azote de semis de Cèdre (Cedrus atlantica Manetti) inoculés en pépinière par Tricholoma tridentinum Sing. var. cedretorum Bon. Ann for sci 58:289–300
Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, Hause B, Bucher M, Kretzschmar T, Bossolini E, Kuhlemeier C, Martinoia E, Franken P, Scholz U, Reinhardt D (2010) Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J 64:1002–1017
Brown JD, Lilleland O (1946) Rapid determination of potassium and sodium in plant material and soil extracts by Flame photometry. Proc Amer Soc Hort Sci 48:341–346
Cardoso IM, Boddington CL, Janssen BH, Oenema O, Kuyper TW (2006) Differential access to phosphorus pools of an oxisol by mycorrhizal and non mycorrhizal maize. Commun Soil Sci Plant Anal 37:1537–1551
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19:292–305
Correia PJ, Gama F, Pestana M, Martins-Loução MA (2010) Tolerance of young (Ceratonia siliqua L.) carob rootstock to NaCl. Agric Water Manag 97:910–916
Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxydation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Di Martino C, Palumbo G, Vitullo D, Di Santo P, Fuggi A (2018) Regulation of mycorrhiza development in durum wheat by P fertilization: Effect on plant nitrogen metabolism. J Plant Nutr Soil Sc 181:429–440
Duponnois R, Colombet A, Hien V, Thioulouse J (2005) The mycorrhizal fungus Glomus intraradices and rock phosphate amendment influence plant growth and microbial activity in the rhizosphere of Acacia holosericea. Soil Biol Biochem 37:1460–1468
Essahibi A, Benhiba L, Fouad MO, Ait Babram M, Ghoulam C, Qaddoury A (2016) Initial nutritional status and exogenous IBA enhanced the rooting capacity of carob (Ceratonia siliqua L.) cuttings under mist system. J Mater Environ Sci 7:4144–4150
Essahibi A, Benhiba L, Ait Babram M, Ghoulam C, Qaddoury A (2018) Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees 32:87–97
Ezawa T, Smith SE, Smith FA (2001) Differentiation of polyphosphate metabolism between the extra-and intraradical hyphae of arbuscular mycorrhizal fungi. New Phytol 149:555–563
Gholamhoseini M, Ghalavand A, Dolatabadian A, Jamshidi E, Khodaei-Joghan A (2013) Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agrc Water Manag 117:106–114
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Goussous SJ, Mohammad MJ (2009) Comparative effect of two arbuscular mycorrhizae and N and P fertilizers on growth and nutrient uptake of onions. Int J Agric Biol 11:463–467
Gyaneshwar P, Naresh KG, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93
Harley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic Press, New York, p 483
Havlin JL, Beaton JD, Tisdale SL, Nelson WL (2005) Soil fertility and fertilizers: an introduction to nutrient management. Pearson Prentice Hall, Upper Saddle River, pp 97–141
Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. College of Agr, Univ of California, Berkeley. p 347
Hori K, Wada A, Shibuta T (1997) Changes in phenoloxidase activities of the galls on leaves of Ulmus vidana formed by Tetraneura funiformis. Appl Entomol Zool 32:365–371
Iniobong EO, Solomon MG, Osonubi O (2008) Effects of arbuscular mycorrhizal fungus inoculation and phosphorus fertilization on the growth of Gliricidia sepium in sterile and non-sterile soils. Res J Agron 2:23–27
Irigoyen JJ, Emerich DW, Sánchez Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plantarum 84:55–60
Javot H, Penmetsa RV, Terzaghi N et al (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci 104:1720–1725
Juszczuk I, Malusà E, Rychter AM (2001) Oxidative stress during phosphate deficiency in roots of bean plants (Phaseolus vulgaris L.). J Plan Physiol 158:1299–1305
Kooten O, Snel JF (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Liu A, Hamel C, Hamilton RI, Smith DL (2000) Mycorrhizae formation and nutrient uptake of new corn (Zea mays L.) hybrids with extreme canopy and leaf architecture as influenced by soil N and P levels. Plant Soil 221:157–166
Makris DP, Kefalas P (2004) Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. Food Technol Biotechnol 42:105–108
Manaut N, Sanguin H, Ouahmane L, Bressan M, Thioulouse J, Baudoin E, Galiana A, Hafidi M, Prin Y, Duponnois R (2015) Potentialities of ecological engineering strategy based on native arbuscular mycorrhizal community for improving afforestation programs with carob trees in degraded environments. Ecol Eng 79:113–119
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London, p 645
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nagarathna TK, Prasad TG, Bagyaraj DJ, Shadakshari YG (2007) Effect of arbuscular mycorrhiza and phosphorus levels on growth and water use efficiency in sunflower at different soil moisture status. J Agr Technol 3:221–229
Naghashzadeh M (2014) Response of relative water content and cell membrane stability to mycorrhizal biofertilizer in maize. EJ Bio 10:68–72
Nagy R, Karandashov V, Chague V et al (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J 42:236–250
Phillips JM, Hayman DS (1970) Improve procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Plénet D, Mollier A, Pellerin S (2000) Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant Soil 224:259–272
Raghuwanshi R, Saxena A (2017) Phosphorus management in agricultural soils by mycorrhizal fungi. In: Aggarwal A, Yadav K (eds) Mycorrhizal fungi. Astral Internati onal Pvt. Ltd, New Delhi, pp 222–244
Sakcali MS, Ozturk M (2004) Eco-physiological behavior of some mediterranean plants as suitable candidates for reclamation of degraded areas. J Arid Environ 57:1–13
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole ecosystem experiment. Proc Natl Acad Sci 105:11254–11258
Shanahan JF, Edwards IB, Quick JS, Fenwick JR (1990) Membrane thermostability and heat tolerance of spring wheat. Crop Sci 30:247–251
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle S, Bottomley P (eds) Methods of soil analysis. Part 2: microbiological and biochemical properties. Soil Science Society of America, Madison, pp 775–833
Turk MA, Assaf TA, Hameed KM, Al-Tawaha (2006) Significance of mycorrhizae. World J Agric Sci 2:16–20
Turkan I, Bor M, Ozdemir F, Koca H (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Walder F, Brule D, Koegel S et al (2015) Plant phosphorus acquisition in a common mycorrhizal network: regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol 205:1632–1645
Wu QS (2017) Arbuscular mycorrhizas and stress tolerance of plants. Springer, Berlin. https://doi.org/10.1007/978-981-10-4115-0
Xie X, Huang W, Liu F et al (2013) Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol 198:836–852
Acknowledgements
The authors acknowledge the Babram society (Marrakesh, Morocco) to provide cuttings and the nursery to prepare the plants used in this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Essahibi, A., Benhiba, L., Fouad, M.O. et al. Responsiveness of Carob (Ceratonia siliqua L.) Plants to Arbuscular Mycorrhizal Symbiosis Under Different Phosphate Fertilization Levels. J Plant Growth Regul 38, 1243–1254 (2019). https://doi.org/10.1007/s00344-019-09929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09929-6