Abstract
A broad body of literature has been published regarding roof-harvested rainwater quality around the world. In particular, the presence of fecal indicator bacteria and pathogenic microorganisms has raised concerns regarding the acceptability of rainwater for potable and non-potable uses. As the use of molecular assays has improved understanding of the diverse microbial communities present in rainwater tanks and their role in providing benefits or harm to human health, a comprehensive review is needed to summarize the state of the science in this area. To provide a summary of microbial contaminants in rainwater tanks and contextual factors, a comprehensive review was conducted here to elucidate the uses of rainwater, factors affecting water quality, concentrations of fecal indicators and pathogens, the attribution of pathogens to host sources using microbial source tracking, microbial ecology, human health risks determined using epidemiological approaches and quantitative microbial risk assessment, and treatment approaches for mitigating risks. Research gaps were identified for pathogen concentration data, microbial source tracking approaches for identifying the sources of microbial contamination, limitations to current approaches for assessing viability, treatment, and maintenance practices. Frameworks should be developed to assess and prioritize these factors in order to optimize public health promotion for roof-harvested rainwater.
Similar content being viewed by others
Introduction
Several studies reported that roof-harvested rainwater (RHRW) for drinking or domestic use has been associated with disease risks and outbreaks.1,2,3,4 Rainwater harvesting is currently being practiced widely and is increasing as a result of a growing awareness of water conservation and stormwater runoff issues, desire for self-sufficiency, the proliferation of environmentally friendly housing developments, and incentives, subsidies, or mandates by government organizations.5 Although numerous global examples of government-initiated drivers are available, pertinent ones are particularly abundant in Australia following the severe “millennium” drought from 2001 to 20096 including the Australian Capital Territory (ACT) Think Water, Act Water Strategy,7 the Australian Queensland Government Home and Garden WaterWise Rebate Scheme,8 and Queensland Development Code MP 4.2—Water Savings Target (QDC MP4.2).9
Given the variable nature of RHRW quality from different roof catchments, it can be challenging to designate appropriate uses in terms of water quality and safety. Despite this limitation, it is important to identify contaminants and contributing factors for guiding rainwater treatment and use. The ultimate goal is to encourage sustainable water use while not promoting a significantly increased exposure to waterborne pathogens associated with negative health outcomes. A typical engineered RHRW collection system relies upon a sloped catchment system (a roof) which drains via a gutter and attached pipe into a storage container (cistern or tank).10 The first flush is typically collected separately while overflow is diverted.11 The remaining collected water in the tank can be pumped either into a treatment system supplying water for domestic use or to an area, where it can be used directly for irrigation or other uses.12 The quality of RHRW may vary according to geographic and catchment locations, climatic conditions, organic material in the gutter, the presence of animal feces, the volume and retention time of the water in the tank, the roof condition, condition of the piping and storage systems, maintenance and management of the system.13
In light of potential exposures to microbiological and chemical contaminants, a number of studies have characterized the quality of RHRW. RHRW contains various contaminants including microorganisms, chemicals, nutrients, and heavy metals, which can cause acute or chronic illness.13,14 Chemicals and metals in RHRW have been reviewed and/or studied previously.13,15,16,17,18,19,20,21 Due to the potential for acute health impacts, especially for immunocompromised populations, this review will focus on only microbial contaminants. In this review, we collated the global abundance of fecal indicator bacteria (FIB) and pathogens in RHRW tank samples. We provide insights into the bacterial communities and sources of fecal contamination in tank water samples. We discuss health risks determined using epidemiological approaches and quantitative microbial risk assessment (QMRA). Finally, we highlight the potential treatment of rainwater to manage the risk of gastroenteritis or other diseases associated with the consumption of RHRW.
Rainwater uses
Some potential RHRW uses scenarios include potable use, non-potable reuse (toilet flushing, household cleaning, clothes washing, lawn irrigation, emergency supplies for fire-fighting, ornamental use), irrigation of produce for consumption, and cooking via installation and maintenance processes.21,22 In developed and developing countries, rainwater harvesting and reuse have been widely practiced for potable and non-potable applications such as irrigation and stormwater management purposes.5 Many regions such as Australia, New Zealand, East Africa, Zambia, China, Singapore, Greece, and Bermuda rely on RHRW as a primary source of water for one or more of these uses.3 Roof-harvested rainwater has been found suitable for drinking in some cases in these areas, although exceedances of health-based guidelines for drinking water have been observed.23,24,25,26,27
Factors affecting RHRW quality
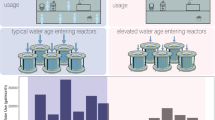
Fecal indicator bacteria and pathogens can enter RHRW tanks through aerosol deposition, tree litter, and animal fecal matter, in addition to indigenous growth in biofilms and sediments.28,29,30,31 While factors associated with FIB have been identified such as local wildlife,32 factors associated with opportunistic pathogens in tank water are less well-characterized and have been linked to duration since previous rainfall for Pseudomonas aeruginosa (P. aeruginosa).33 In premise plumbing, elevated opportunistic pathogen concentrations have been linked to high water age, lack of disinfectant residual, elevated temperatures, and pipe material.34 There is limited information available regarding differences in microbial ecology or pathogen occurrence in premise plumbing fed by rainwater tanks.35 However, such information would be useful for informing pathogen control practices.
With regard to FIB and enteric pathogens, roof materials associated with the construction of RHRW systems are particularly important for water quality. A comparison of asphalt fiberglass shingle, metal, concrete tile, cool, and green roof materials showed that the first flush from metal roofs tended to have lower concentrations of FIB.36 This is because FIB were completely washed away by the first flush diverter. Lee and colleagues37 found metal roofs to be the most suitable for RHRW based on total suspended solids, nutrients, metals, and Escherichia coli (E. coli) concentrations. However, metal roofs can also contribute to increasing the load from dissolved metals, and an optimal roof for preventing contamination has not yet been identified.13 Mendez and colleagues36 noted that regardless of roof material (asphalt fiberglass shingle, Galvalume metal, and concrete tile were compared), rainwater would need treatment in order to meet potable and non-potable guidelines in the US. Limited studies were available regarding full-scale vegetated “green” roofs, and there is not a consensus regarding the ideal choice of green roof design, media, or vegetation for maximizing water quality.
Meteorological parameters may influence bacterial composition of RHRW due to airborne microorganisms. For example, results indicated that the bacterial load in RHRW is directly affected by wind speed while the composition of microorganisms varied with wind direction.29 This could be due to greater uplift of organisms from sources and arrival of more organisms at the roof catchment surface per unit time.29 During dry antecedent periods, dust, aerosols, and gases from the atmosphere can be directly transferred by deposition to tanks if openings exist.19 The duration of dry antecedent conditions plays a role in the amount of accumulated contaminants that are washed from roofs into rainwater tanks. Longer dry antecedent periods are generally associated with higher bacterial counts due to accumulation of debris, organic matters and fecal droppings on the roof catchment.19,33,38,39,40
Concentrations of Escherichia coli and pathogens
Escherichia coli
Drinking water guidelines are used to assess the microbial quality of the RHRW when used for drinking. Guidelines such as World Health Organization (WHO), Canadian and the Australian drinking water guidelines state that E. coli should not be detected in a 100 mL sample of drinking water, and if detected, immediate action should be taken to minimize human health risks.5 E. coli are commonly found in the feces of warm-blooded animals in high numbers.41 Therefore, their presence in a water source indicates the presence of fecal contamination and potential enteric pathogens. Most research studies on RHRW reported to date used E. coli to assess the microbiological quality of the water.
Table 1 shows the concentrations of E. coli in RHRW tank samples in the research literature. The percentage of positive samples in rainwater tanks ranged from 24 to 92%, and therefore all studies had at least one tank that exceeded WHO health-related drinking water guidelines (absence of E. coli per 100 mL). Ranges of concentrations were variable, ranging up to 10,000 colony forming units (CFU)/100 mL in a study from Malaysia42 and 10,964 gene copies (GC)/100 mL in an Australian study.43 High concentrations were also observed in Bangladesh (6000 CFU/100 mL)44 and other Australian studies (~103 CFU/100 mL)1,45,46. The variability in E. coli concentrations observed in the studies has been linked to meteorological factors,33 catchment location, roof and/or storage container materials,36 the presence of wildlife near the roof,32 laboratory method used, and location and/or timing of the sampling event relative to the previous factors.33
Rainwater tanks are commonly maintained by individual owners, therefore, often data are not available in order to assess the water age or hydraulic factors for specific tanks. Data from nearby rain gauges can sometimes be far from the actual tank, resulting in a loss in highly localized information and factors that are relevant to rainwater contamination. Among studies, rainwater sampling points are not standardized, and limited meta-data is available for evaluating relationships with the aforementioned factors. Sharing of open datasets is recommended for encouraging meta-analysis of RHRW contamination datasets.47 Specifically, with regard to comparing quantification methodologies, differences in culture-based vs. molecular approaches are known to provide varying information regarding cell concentrations. Culture-based methods for enumerating E. coli were utilized in all studies, and one study supplemented culture-based measurements with qPCR-based GC/L.43 Among the culture-based methods, plating membrane filters (15 of 18 studies) spread plate methods (1 of 18 studies), and Colilert Quanti-Tray (IDEXX) (3 of 18 studies, 1 using both membrane filtration and IDEXX) were used. To highlight some of the factors that differed among available studies, a brief description of the studies summarized in Table 1 is provided below.
Sazakli and colleagues26 determined the concentrations of E. coli in RHRW tank samples in Kefalonia Islands, Greece. During a three year survey, 156 RHRW samples were collected from 13 tanks over 12 seasonal events. Among the 156 samples tested, 41% were positive for E. coli with concentrations ranging from 0 to 250 CFU/100 mL of water. E. coli exhibited seasonal variations, and the highest percentage of positive samples was detected in autumn while in winter it was decreased and was gradually increased in spring and summer. The authors suggested that examination of rainwater quality from a microbial point of view is a prerequisite before its utilization for drinking.
Levesque and colleagues48 assessed the contamination status of RHRW tanks used for drinking throughout Bermuda. Of the 102 tank water samples, 66% were found to be contaminated with E. coli regardless of the method used. The concentrations of E. coli ranged from 1 to>100 CFU or MPN/100 mL of water. The authors raised concerns regarding the E. coli contamination as the residents used the water for drinking without prior boiling or other forms of disinfection and or filtering treatment.
Ample data is available on the concentration of E. coli in RHRW tank samples from Australasia. In 2010, Ahmed and colleagues1 screened 100 RHRW tank samples for E. coli and a range of enteric pathogens and opportunistic pathogens. Of the 100 samples tested, 58% were positive for E. coli, and the concentrations ranged from 1 to 3060 CFU/100 mL water. At least 5% of the tested tank water samples had E. coli levels exceeding 1000 CFU/100 mL of water.
Ahmed and colleagues43 determined the concentrations of E. coli in 50 RHRW tank samples using culture-based and quantitative PCR (qPCR) methods simultaneously. Among the 50 samples tested, 52 and 92% of the samples were positive for E. coli as determined by culture-based and qPCR methods, respectively. The concentrations of E. coli ranged from 3 to 2290 CFU (for culture-based methods) and 22 to 10,964 GC/100 mL of water. The concentrations of E. coli measured by the qPCR methods were 1–2 orders of magnitude higher than culture-based methods. Ahmed and colleagues49 also determined the concentrations of E. coli in 24 RHRW tank samples and corresponding household drinking water taps in the SEQ region. Among the 24 samples tested from the rainwater tanks, 62% were positive (concentrations ranged from 1 to 230 CFU/100 mL) for E. coli, whereas 58% tap water samples were also positive (concentrations ranged from 1 to 300 CFU/100 mL) for E. coli. The concentrations of E. coli were not significantly different in drinking tap water samples compared to the tank water samples indicating the inefficacy of the under sink filtration method used.
Hamilton and colleagues46 tested 134 RHRW tank samples from SEQ for the presence of E. coli and a number of opportunistic pathogens. Among the samples tested, 68% were positive for E. coli and the concentrations ranged from 0 to 2420 MPN/100 mL water. A follow-up study by the same group determined the seasonal abundance of E. coli along with Enterococcus spp. and a number of opportunistic premise plumbing pathogens in RHRW tanks in SEQ.33 A total of 24 RHRW tanks were repeatedly sampled over six monthly events. Among the 144 tank water samples, 44% were positive for E. coli with concentrations ranging from 1 to 687 MPN/100 mL of water. Seasonal differences were observed for E. coli prevalence during the wet and dry season. The average concentrations of E. coli were higher in the wet season than the dry season.
Abbott and colleagues50 investigated the microbiological quality of tank water samples of 560 private dwellings in New Zealand. At least half of the samples analyzed exceeded the acceptable standards for contamination and in 41% of the samples had evidence of fecal contamination.
In South Africa, Dobrowsky and colleagues51 analyzed 80 RHRW tank samples from ten tanks representing ten houses from a cluster of 411 houses for the presence of E. coli on eight separate events representing low and high rainfall periods. During the low rainfall periods, 44% of the tank water samples exceeded the South African Department of Water Affairs (DWAF) guidelines. However, during the high rainfall period, more tanks (79%) exceeded the guideline value. The concentrations of E. coli ranged between 0 and 250 CFU/100 mL of water.
Evidently, plenty of data are available on the concentrations of E. coli in RHRW stored in tanks. However, data on the concentrations of E. coli in fresh rainwater is scarce. Kaushik and colleagues52 determined the concentrations of E. coli in fresh rainwater samples in the tropical urban environment of Singapore using qPCR assays. Among the 50 samples tested, 21 (42%) samples contained E. coli with as high as 14,000 GC/100 mL of rainwater. The authors suggested that E. coli in fresh rainwater may have derived from bioaerosols. Cloud and rain droplets are known to scavenge atmospheric aerosols and gases through nucleation and below cloud scavenging mechanisms, respectively. It is thus quite likely that the microbial pathogens or flora associated with aerosol particles incorporated into rainwater through such scavenging mechanisms.53 In a later study, Kaushik and colleagues54 also determined the concentrations of culturable E. coli in fresh rainwater samples from four sites for four months. Of the 15 samples tested, 81% were positive for E. coli with concentrations ranging from 0 to 75 CFU/100 mL of water.
The reliability of E. coli as a health based indicator for monitoring RHRW quality has been questioned due to their poor correlations with pathogens (Ahmed et al.1). However, E. coli can still be useful in determining seasonal changes in water quality as well as useful for assessing the sources of treatment efficacy, preventative maintenance and design of the system.
Pathogenic microorganisms in RHRW stored in tanks
A recent article on the risk-based enteric pathogens log reduction targets for non-potable and potable uses of gray water, stormwater and RHRW, reported that log reduction targets for RHRW remain uncertain due to lack of data on enteric pathogens.55 The following section and Table 2 summarizes key research articles that reported the occurrence of enteric pathogens, opportunistic pathogens, and other clinically significant microorganisms in water samples associated with RHRW tanks worldwide.
Pathogens found in rainwater include members of genus Campylobacter spp., Salmonella spp., Shigella spp., Vibrio spp., pathogenic E. coli, Acinetobacter spp., Aeromonas spp., Citrobacter spp., Klebsiella spp., Legionella spp., Mycobacterium spp., Pseudomonas spp., Staphylococcus spp., Yersinia spp., Cryptosporidium spp., Giardia spp., Isospora spp., adenoviruses, Acanthamoeba spp., Naegleria spp., Entamoeba spp., and Endolimax spp.
Comparing data across pathogen studies is more challenging than FIB because of a variety of laboratory methods utilized among the studies and similar considerations as discussed for E. coli regarding lack of available meta-data reported regarding sampling location, roof material, and other factors. The number of positive samples for various pathogens ranged from 0.54 to 98%. Legionella spp. has the most common pathogen-containing genus measured from an Australian study.46 Ranges in pathogen concentrations varied considerably, with P. aeruginosa having the highest concentration of any pathogen in an Australian study using qPCR for quantification.33 The high variability in pathogen occurrence is attributable to numerous factors including pathogen source (fecally-attributed vs. common resident of environmental matrices such as opportunistic pathogens Legionella spp. and Mycobacterium spp.), time since introduction of animal fecal materials, degree of presence of wildlife in the roof/tank area, hydraulic performance of the tank, and ambient conditions. Studies summarized in Table 2 are described in further detail below.
Daoud and colleagues56 determined the prevalence of 11 enteric and opportunistic pathogens in 42 RHRW tank samples collected during summer and winter seasons in West Bank, Palestine using PCR. Among the 11 pathogens tested, 5 were detected in tank water samples at various frequencies. Citrobacter spp. [Citrobacter freundii (C. freundii) and Citrobacter braakii (C. braakii)] were detected in 83% of the samples, whereas, Acinetobacter spp., A. hydrophila, Pseudomonas aeruginosa (P. aeruginosa) and Campylobacter spp. (C. jejuni and C. coli) were detected in 78, 52, 7, and 7% of the samples, respectively. The prevalence of pathogens was higher in samples collected in summer compared to those collected in winter. Based on the occurrence of pathogens in Palestinian RHRW tanks, the authors concluded that RHRW should not be consumed without proper treatment that improves the quality of this water to potable water quality.
Ahmed and colleagues49 investigated the quantitative occurrence of Campylobacter spp. and Salmonella spp. in water samples from 24 RHRW tanks and 24 corresponding connected household taps in SEQ using qPCR. Amongst the 24 households, 21% of the RHRW and 21% of the tap water samples contained Campylobacter spp., respectively. The tap water samples did not contain any Salmonella spp., however, 4% of the RHRW samples contained Salmonella spp. The numbers of Campylobacter cells in RHRW household tap water samples ranged from 5 to 110 (in RHRW) and 12 to 19 (in tap water) cells/L of water. Similarly, the estimated number of Salmonella cells was 7300 (in RHRW)/L of water.
Hamilton and colleagues46 tested 134 RHRW tank samples from SEQ for the presence of seven potential opportunistic pathogens using qPCR. Of the 134 samples tested, 9.7, 98, 3, 17.2, 78.4, and 28.4% were positive for Acanthamoeba spp., Legionella spp., L. pneumophila, Mycobacterium avium (M. avium), Mycobacterium intracellulare (M intracellulare), and P. aeruginosa, respectively. The concentrations of Legionella spp., P. aeruginosa, M. intracellulare, Acanthamoeba spp., M. avium and L. pneumophila in positive samples ranged from 300 to 310,000, 310 to 96,000, 23 to 68,000, 210 to 66,000, 22 to 11,000 and 240 to 980 GC/100 mL of water. The authors suggested that these potential opportunistic pathogens in tank water may present health risks from both potable and non-potable uses.
A follow-up study by the same group determined the seasonal abundance of six opportunistic pathogens in RHRW tanks in SEQ.33 A total of 24 RHRW tanks were repeatedly sampled over six monthly events. Among the 144 tank water samples, 40, 97, 5, 57, 60, and 31% were positive for Acanthamoeba spp., Legionella spp., L. pneumophila, M. avium, M. intracellulare and P. aeruginosa, respectively. The concentrations of P. aeruginosa in positive samples were 360 to 470,000,000 GC/100 mL, followed by Legionella spp. (320–2,300,000 GC/100 mL), Acanthamoeba spp. (220–980,000 GC/100 mL), M. intracellulare (22–680,000 GC/100 mL), M. avium (24–360,000 GC/100 mL) and L. pneumophila (23–150 GC/100 mL). The authors noted some seasonal differences between the prevalence of opportunistic pathogens during the wet and dry seasons. Legionella spp. and M. intracellulare were consistently present across sampling events, but both occurred at higher concentrations during the dry season. In contrast, P. aeruginosa concentrations peaked in the wet season, and L. pneumophila were only detected in the wet season. The authors concluded that infection risks might exceed commonly cited benchmarks for uses reported in the rainwater usage survey (such as pool top up) and warrant further exploration through QMRA.
Ahmed and colleagues57 screened a collection of E. coli isolates from 22 RHRW tank samples in SEQ, Australia and tested for the presence of 20 virulence genes. Of the 22 rainwater tanks, 36 and 23% were positive for the eaeA and ST1 genes, respectively. Extraintestinal pathogenic E. coli (ExPEC) virulence genes cdtB, cvaC, ibeA, kpsMT allele III, PAI, papAH, and traT were detected in 68% tank water samples. The authors concluded that public health risks associated with clinically significant E. coli in RHRW tanks should be assessed.
Dobrowsky and colleagues51 analyzed 80 RHRW tank samples from 10 tanks representing 10 houses for the presence of pathogenic E. coli. The authors isolated 92 E. coli from the tank water samples. Of these isolates, 6% were identified as E. coli O157:H7 and 4% were positively identified as enterotoxigenic E. coli (ETEC) using 16S rRNA. In addition to screening the isolates, the authors also tested the tank water samples for the detection of pathogenic E. coli (EPEC, EIEC, EHEC, and EAEC). The aggR gene of EAEC pathotype was more frequently detected than EHEC and EPEC.
Limited data are available on the presence of protozoa pathogens in RHRW (Table 2). Crabtree and colleagues58 found that 45 and 23% of the 44 private and public tank water samples in U.S. Virgin Islands were positive for Giardia cysts and Cryptosporidium oocysts, respectively. The levels of cysts and oocysts were found to range from 1 to 10 organisms/100 L of water, with one sample containing 70 oocysts/100 L. Simmons and colleagues59 also reported the presence of Cryptosporidium spp. in 4% of tank water samples in Auckland, New Zealand. Albrechtsen60 similarly reported the presence of Cryptosporidium spp. in Danish RHRW. They tested 17 rainwater samples, of which six were positive for Cryptosporidium spp. The numbers of Cryptosporidium spp. were as high as 50 oocysts/L of water. Another study in Queensland, Australia, reported the presence of G. lamblia in 19% of RHRW samples tested, but none of the samples were positive for Cryptosporidium parvum.1,61
While data exists on certain bacterial and protozoa pathogens present in RHRW, data on the occurrence of enteric viruses in RHRW tank samples are scarce. Waso and colleagues62 reported the presence of a number of microbial and chemical source tracking markers in RHRW tank samples from the Kleinmond Housing Scheme site, Kleinmond (Western Cape), South Africa. The authors randomly selected 10 RHRW systems from the housing scheme and collected 40 tank water samples and 40 gutter debris samples over 4 events. Human adenovirus was detected in 42.5 and 52.5% of the RHRW and gutter debris samples, respectively at levels ranging from below the detection limit to 316 and 1253 GC/µL of DNA in tank water and debris samples, respectively. The presence of adenovirus in tank water and gutter debris samples was also supported by the co-occurrence of Bacteroides HF183, salicylic acid, and caffeine. The authors concluded that the presence of the human adenovirus indicates that there are health risks associated with the consumption of the harvested rainwater and the water may not be suitable for potable purposes without prior treatment.
Most of the studies in literature determine the pathogen (including opportunistic) concentrations in tank water samples, while little is known regarding the presence of opportunistic pathogens in biofilms inside the tanks. Al-Bahry and colleagues63 investigated the prevalence of several opportunistic pathogens in three different types of tanks (i.e., galvanized iron, polyethylene and glass-fiber reinforced plastic). In all, 30 biofilm samples were collected from the internal surface of the tanks, shower heads and inner tap faucets to determine the presence of opportunistic pathogens. The highest frequency of Aeromonas spp. was detected in galvanized tanks (77.5%) compared with the glass-fiber reinforced plastic tanks (26.3%) and polyethylene tanks (29.6%). Aeromonas sobria and A. hydrophila were isolated from these tank biofilm samples. Pasteurella spp. (namely Pasteurella haemolytica and Pastereulla pneumotropica) were detected in 17.2% of the glass-fiber reinforced plastic and 23.4% of the galvanized iron tanks. Pathogenic Pseudomonas pseudomallei was isolated in low frequencies in all tanks. Salmonella typhimurium and Salmonella arizonae were detected in the galvanized iron and glass-fiber reinforced plastic tanks. The authors concluded that physico-chemical factors could promote microbial growth and have a significant effect on water quality. Therefore, a high level of inspection would be required by the relevant government authorities to ensure the water is safe for its designated use. It is known from studies of drinking water distribution systems and storage tanks, that biofilms form on wetted surfaces and can harbor human-pathogens.64 Therefore, future work might investigate the microbial diversity of biofilms in rainwater tanks along with their corresponding ability to harbor human pathogens and their association with hydrologic/climate parameters, design, and maintenance schemes. Factors that influence the levels of pathogens in biofilms include turbidity, concentrations of organic carbon and biodegradable organic carbon in the water, biofilm particle surface properties, pipe material, flow rates, and water treatment schemes.65,66,67
Microbial communities of roof-harvested rainwater
Brodie and colleagues28 described the microbial diversity of urban aerosols, finding over 1800 bacterial species via DNA microarray in aerosols from two sites in San Antonio and Austin, Texas, including organisms in the human pathogen-containing families Campylobacteraceae and Helicobacteraceae, indicating a potential for microbial deposition of human pathogens on RHRW catchment surfaces.
Despite numerous observations of the indicator and pathogenic microorganisms in the RHRW literature, the microbial ecology of domestic rainwater systems and storage tanks has been studied to a lesser extent. Although no North American/European examples are available, Evans and colleagues68 assessed the bacterial diversity within 22 domestic Australian rainwater storage tanks (n = 83 samples) over a two year period, finding generally high diversity similar to soil and seawater. A combination of culture and PCR analysis revealed 202 different species, with the majority (90%) from the phyla Proteobacteria (94% of the samples, average abundance >5000 CFU/mL of water), Firmicutes (70% of the samples), Actinobacteria (27% of the samples), and Bacteroidetes (24% of the samples).68 Coliform bacteria and species associated with fecal contamination were associated with <15% of the identified species and <1.5% of the total average abundance in rainwater tanks.68 Despite the presence of several potential pathogenic species, the authors concluded that rainwater tanks might support functional ecosystems comprising complex communities of environmental bacteria which may have beneficial implications for the quality of harvested rainwater.
The recent advances and reduced costs of high throughput sequencing have accelerated the analysis of microbial communities in a complex microbial ecosystem.69 To the best of our knowledge, only two studies have investigated the potential application of sequencing to determine microbial community structure in RHRW tank samples. Chidamba and Korsten70 applied pyrosequencing to determine microbial community structure in seven RHRW tank samples collected from a rural village in the Eastern Cape Province of South Africa. Results from the study indicated that Burkholderiaceae, Comamonadaeceae, Oxalobacteraceae, Planctomycetaceae and Sphingomonadaceae were the most abundant families in the collected RHRW tank samples. Additionally, signatures of pathogenic bacteria belonging to the genera Chromobacterium, Clostridium, Legionella, Serratia, and Yersinia were detected in the tank samples.
Recently, Ahmed and colleagues71 used an Illumina-based next-generation sequencing (NGS) approach to monitor the abundance and diversity of bacterial communities in RHRW in SEQ [88 samples collected from Brisbane (representative of an urban area) and Currumbin (representative of a peri-urban area)]. Similar to the results obtained by Chidamba and Korsten,70 at the family level, Comamonadaceae and Planctomycetaceae were the most abundant families, followed by Chitinophagaceae and Parachlamydiaceae.
Using a principal component analysis, the authors also noted that there was a separation of taxa between the urban and peri-urban areas. In contrast to the low number of signatures of pathogenic genera detected by Chidamba and Korsten (n = 5), Ahmed and colleagues71, identified 34 potentially pathogenic genera in the collected RHRW samples, including Acinetobacter, Bordetella, Burkholderia, Legionella, Mycobacterium, Pseudomonas, Rickettsia, and Tatlockia.
Although there are only a handful of studies investigating the bacterial community structure in RHRW stored in tanks, information on the microbial communities in fresh rainwater is even scarcer. Kaushik and colleagues54 constructed clone libraries from composite DNA samples of fresh rainwater and reservoir water at four sites in Singapore. The authors detected 10 classes of bacteria in fresh rainwater and four classes in reservoir water. In fresh rainwater, sequences were affiliated with Betaproteobacteria, Alphaproteobacteria, Sphingobacteria, Actinobacteria, Gammaproteobacteria, Lentisphaerae, CH21, Phycisphaerae, Chlorbia, and Spirochaetes. In contrast, the reservoir water library detected sequences affiliated with only Betaproteobacteria, Alphaproteobacteria, Sphingobacteria, and Gammaproteobacteria. The fresh rainwater had higher diversity and taxonomic richness at the class level than those of reservoir water. Betaproteobacteria dominated both the communities. The OTU with the greatest difference in the relative abundance for fresh rainwater was Curvibacter, whereas for reservoir water it was Ralstonia. The authors suggested undertaking high throughput sequencing to obtain more information on the bacterial community structure in fresh rainwater due to the low coverage of traditional cloning methods.
A recent study also used pyrosequencing of the ITS 1 and ITS 2 hypervariable regions of the 18S rRNA gene to investigate fungal community structure in five RHRW tank samples collected from rural villages in South Africa.72 At phylum level, fungal sequences were classified into five phyla. These include Ascomycota, Basidiomycota, Chytridiomycota, Glomeromycota, and Zygomycota. Ascomycota dominated the dataset while 45.7% of the sequences were not classified to any known fungal phyla. The authors also noted significant differences in fungal diversity in the selected tank water samples. Classification at the species level revealed a diverse fungal population. Sordariomycetes spp., Davidiella tassiana, Dothideomycetes spp., Tremellales spp. and Knufia Perfornans Sterflinger were identified as dominant species. Since human pathogenic yeasts such as Cryptococcus spp. were detected in RHRW tank samples, further investigation may be required to identify the effect of continuous exposure on the health of the end-users.
Microbial community analysis although rarely used to monitor the quality of RHRW, it can provide information on the overall quality of tank water. In addition, this approach can be used as a broad screening step to identify potential pathogens to target using more sensitive molecular methods such as qPCR to better protect public health.
Microbial source tracking in roof-harvested rainwater
Microbial water quality is generally monitored using FIB such as E. coli and enterococci.1,26,29,61,73,74. Studies have shown, however, that the presence of these indicator groups does not always correlate well with the presence of pathogens in water sources.74 Indicators, which consistently correlate with the presence of pathogens and which identify specific sources of contamination thus need to be identified.74 Current research, therefore, focuses on identifying, validating and applying microbial source tracking (MST) markers as alternative or supplementary fecal indicators for the monitoring of water quality. These markers have been applied to various water sources, however, limited information on their presence in RHRW is available.
Ahmed and colleagues32 screened RHRW for the presence of possum (PSM) (Trichosurus vulpecula) and avian (GFD) associated MST markers to determine whether these animals contribute to the fecal contamination of RHRW in Australia. In total, 134 RHRW samples were collected from tanks in Brisbane (n = 84) and the Currumbin Ecovillage (n = 50) in SEQ. Of the 134 rainwater samples collected, 29.1% (n = 39; 370–85,000 × 105 GC/L) were positive for the GFD marker, while 8.2% (n = 11; 2000–6800 GC/L) tested positive for the PSM marker. As avian and possum associated markers were detected in RHRW, a potential link between the decline of the microbial quality of the RHRW and avian and possum feces was established. Ahmed and colleagues32 also noted that the regular cleaning of rooftops and gutter systems and installing devices to prevent birds from perching on rooftops, will greatly reduce fecal contamination on the catchment area and subsequently in the RHRW.
Waso and colleagues62 screened 40 RHRW and 40 gutter debris samples (collected from the Kleinmond Housing Scheme, South Africa) for the human-associated Bacteroides HF183 marker and adenovirus to determine if gutter debris was contributing to the contamination of the RHRW. Overall, Bacteroides HF183 was detected in 57.5% (n = 23) and 95% (n = 38) and adenovirus in 42.5% (n = 17) and 52.5% (n = 21) of the rainwater and gutter debris samples, respectively. In addition, human adenovirus was detected by qPCR in 5% (n = 2) of the RHRW and 22.5% (n = 9) of the gutter debris samples (ranging from below the detection limit to 316 and 1253 CG/µL DNA). Concurrence analysis indicated that the HF183 marker co-occurred in 57.5% of the RHRW and the corresponding rooftop debris samples, while adenovirus co-occurred in 27.5% of the RHRW and corresponding gutter debris samples. Although the HF183 marker and human adenovirus are generally associated with human fecal contamination, the authors hypothesized that animals such as birds might serve as the vectors of these MST markers in the environment. It was also highlighted that debris washing into the tanks during rain events was contributing to the decline of the RHRW quality.
In a follow-up study, Waso and colleagues75 screened 60 RHRW samples for a range of MST markers (Bacteroides HF183, Bifidobacterium adolescentis, human, bovine and porcine mitochondrial DNA, adenovirus and Lachnospiraceae) using qPCR and indicator organisms using culture-based (E. coli, enterococci, heterotrophic bacteria, total, and fecal coliforms) and molecular techniques (qPCR; E. coli and Enterococcus spp.). Various positive correlations were observed between the concentrations of the MST markers and the indicator organisms detected in the RHRW samples. For example, the HF183 marker positively correlated with E. coli (qPCR; p = 0.037), while adenovirus positively correlated with E. coli (culture-based; p = 0.000). Furthermore, 100% concurrence was observed for HF183, adenovirus, and Lachnospiraceae vs. E. coli (qPCR) and enterococci (qPCR), amongst other concurrence frequencies observed. The authors concluded that a variety of MST markers were present in the RHRW. Therefore, fecal contamination of the RHRW was likely, which corresponded to the detection of FIB in the samples. Furthermore, based on the correlations and concurrence frequencies observed, the authors concluded that the HF183 marker, adenovirus and Lachnospiraceae may be utilized to supplement FIB analysis during future screenings.
In summary, results obtained in these studies indicate that MST markers correlate well with indicator organism analysis and assists in identifying the primary contamination sources of RHRW systems.32,62,75 Understanding the source of contamination is crucial in the development of contamination prevention strategies and allows for the accurate estimation of the human health risk associated with utilizing a particular water source.
Human health risks
Clostridium botulinum, Campylobacter spp., S. typhimurium, L. pneumophila, M. avium complex (MAC), Naegleria spp., Salmonella arechevalata, Cryptosporidium, and Giardia have previously been implicated in outbreaks or disease cases associated with RHRW.2,4,76,77,78 In light of these outbreaks, several studies have explored the degree to which RHRW might present microbiological health risks. These studies generally fall into the category of epidemiological studies or quantitative microbial risk assessment (QMRA).
Epidemiological studies
To investigate the relationship between tank water consumption and gastroenteritis in South Australia, a prevalence survey of 9500 four year-old children was undertaken. The survey was followed up with a longitudinal cohort study of gastroenteritis among 1000 four to six-year-old children, selected on the basis of tank water consumption.79 This study found that children drinking tank water were not at a greater risk of gastroenteritis than children drinking supply water. One important limitation of this study was that the majority of the children had consumed tank water for at least one year. Hence an alternative explanation to there being no increased risk associated with tank rainwater was that the children were exposed to potentially low levels of contaminants and may have developed immunity to some organisms. Furthermore it should be noted that no microbial water quality monitoring was done in this study and that 77% of roof catchments were reported to be free of overhanging trees and 65% of gutters had been cleaned in the last year during the study period.
In a double-blinded, randomized controlled trial study of water treatment filters and gastroenteritis incidence among 300 households in Adelaide, Rodrigo and colleagues80 reported that the consumption of untreated rainwater did not contribute appreciably to community gastroenteritis. However, as the authors point out their findings may not be applicable to susceptible and immunocompromised persons, young children, or the elderly because these groups were specifically excluded from their study. Another limitation of study was the lack of an alternative water source control group since all the participants consumed tank water. Of additional concern was the reported high dropout rate (31%) of participants. This may have contributed to the underestimation of the true incidence of gastroenteritis. While the authors state that they conducted limited water quality testing on rainwater tank samples it would have been useful to see if there was any correlation between the E. coli levels, episodes of gastroenteritis, and their severity.
Several epidemiologic studies have sought to evaluate the linkages between RHRW use and illness in a variety of comparative contexts. A systematic review and meta-analysis of eight epidemiological studies2,79,81,82,83,84,85,86 of RHRW-related gastrointestinal illness in Australia, Brazil, Kenya, New Zealand, and Vietnam found no significant difference in risk for consumption of rainwater compared to improved water supplies (according to the World Health Organization definition of an “improved water source” as one that is likely to be protected from outside contamination and/or fecal matter); a lower risk was calculated for consumption of RHRW compared to “unimproved” water supplies.87 It is noted that one of the four studies examining RHRW vs. improved supplies from New Zealand2 indicated a greater risk of campylobacteriosis in a case–control study of drinking rainwater compared to alternative sources based on a small number (23 cases and 11 controls) of the total study participants (621 case-patients and 621 matched control) with rainwater as the source of their home water supply. Four of the five outbreak studies reviewed by Dean and Hunter87 reported that outbreaks were “strongly associated with rainwater use”.4,78,88,89 Importantly, one of the reviewed studies of high (epidemiological) methodological quality85 indicated that RHRW systems with filters did not reduce the risk of illness.87
Since 2012, several epidemiological studies on rainwater consumption have been conducted. In the Dominican Republic, a study of consumption of water from various sources (coded as rainwater, bottled water, or “all other sources”) and diarrheal disease was conducted in children under five years of age from 2002 to 2007 from the Demographic and Health Surveys database.90 Using a binary logistic regression, the authors concluded that consuming bottled water was associated with a lower odds of diarrhea in children under age five compared with rainwater, however this result was not significant (p = 0.25). People who consumed “other sources” of water were 1.34 times more likely to have reported diarrhea in children under five compared to rainwater (combined 2002 and 2007 data). These results are consistent with findings by Dean and Hunter obtained from a cross-sectional observational study in rural Trinidad.91 While epidemiological studies suggest that rainwater is safer compared to unimproved water supplies, when compared to treated drinking water supplies, large epidemiological studies would be needed in order to detect small effect sizes that could result from exposure to rainwater. For example, spatial and temporal heterogeneity in rainwater use and maintenance, lack of consideration of immune factors that might modulate rainwater risks, and underreporting of rainwater-associated illnesses could play a role in creating a gap between epidemiological and microbiological findings.
In a study of diarrheal illness among individuals with and without RHRW tanks in northeast Brazil, Marcynuk and colleagues84 assessed the 30-day period prevalence of diarrhea for 3,679 people from 774 households following the institution of the 2011“One Million Cisterns” project that has provided 351,000 RHRW cisterns to families throughout the semi-arid region of northeast Brazil as of 2011.84 People residing in households with cisterns had a significantly lower 30-day period prevalence (11%) of diarrhea than people from households without a cistern (18%) with the trend remaining in a subgroup analysis of children under five. Children under five had a prevalence of 15.6% compared to 26.7% in children without a cistern. These findings also support that RHRW can provide a better alternative than “unimproved” sources; the rainwater was not compared to consumption of “improved” or treated drinking water sources in the study.
Another study from the same region of Brazil92 investigated the role of rainwater harvesting cisterns in the occurrence of Giardia duodenalis infections in children compared to other children living in households supplied by other water sources. In a quasi-experimental study, a sample of 664 (332 in cistern group, 332 in “other water sources” group) four-month to five-month old children were followed up for one year (2010) and feces were analyzed three times. Giardia risk was higher for children without access to cisterns compared to children who had access. The prevalence ranged from 4.8 to 10.5% in the cistern group compared to 7.6–16.7% in the “other water sources” group92, supporting the conclusion that RHRW can provide a favorable alternative to unimproved water sources.
Pham-Duc and colleagues93 conducted a study of diarrheal diseases among an adult agricultural community population in Hanam province, Vietnam with high wastewater and excreta re-use (n = 867). A nested case-control study (n = 232 pairs of cases and controls) was used to assess risk factors for diarrheal episodes including use of rainwater for drinking, use of local pond water, composting of human excreta, handling human excreta in field work, handling animal excreta in field work, lack of protective measures while working, never or rarely washing hands with soap, and eating raw vegetables the day before. The cohort was followed weekly for 12 months to determine the incidence of diarrhea, and it was determined that the incidence rate was 0.28 episodes per person per year (pppy) at risk. The use of rainwater for drinking (87% of the surveyed residents reported this usage) was significantly associated with diarrheal disease and accounted for 77% of diarrheal episodes. This was furthermore the second most important risk factor after lack of protective measures while working. The authors attributed the relationship between RHRW use and diarrhea cases in the study to the presence of a “sludge layer” on roofs and gutters at sites during household visits. Additional contributing factors suggested were that rainwater was collected from above-ground containers without lids or with an infrequently closed lid using bare hands or with an iron or rubber bucket that was placed on the ground; and that domestic animals such as chickens or other birds may defecate on roofs.
A case-control study was conducted in July 2014 in response to a large 2011 diarrheal outbreak (n = 244 cases) that occurred concurrently with a La Niña-associated drought emergency on the Pacific island nation of Tuvalu, to identify factors that contributed to epidemic transmission.94 The population is highly dependent on RHRW for potable water as well as government or community tanks filled with water treated with reverse osmosis. Seventy-five randomized case subjects were selected for administering a household survey and enrolled. Households with RHRW tank levels below 20% and decreased handwashing frequency were associated with increased risk of diarrhea. The authors propose that the rainwater association could be due to drought conditions limiting the availability of (cleaner) rainwater, and that households may switch to untreated or less hygienic sources; low residual volume in RHRW tanks may concentrate pathogens or reduce the ability to dilute introduced pathogens, increasing the likelihood of consuming a greater dose; or increasing the contaminant load during prolonged dry spells in between first flushes.94
The findings from epidemiological studies indicate that RHRW can provide a decreased risk of diarrheal illness compared to consuming alternative or unimproved water sources. However, when risks are evaluated for RHRW relevant to other sources (such as “improved” water sources), this comparison is contingent upon the quality and context of the alternative water source and does not provide information on absolute risk and its relationship to risk acceptability benchmarks. It is noted here that in epidemiologic and outbreak investigation studies, examining the relationship between exposures to waterborne pathogens and disease can be complicated by multiple factors. These limitations include the underreporting of gastrointestinal or other waterborne illnesses, inability to assess the exposure “denominator” or the total number exposed to a water source of interest during the period of contamination, potential for exposure to the pathogen of interest to occur via multiple media (water, food, etc.), infrequent/intermittent or population-specific illnesses rather than outbreaks (especially for opportunistic pathogens), transitory exposures that have likely passed by the initiation of the outbreak investigation, and lack of environmental sampling during the exposure period to verify matching of environmental strains with clinical outbreak isolates. As a result, alternative methods of quantifying risk due to exposure to pathogens occurring in the environment are often used, such as quantitative microbial risk assessment (QMRA) with a process of hazard identification, exposure assessment, dose-response, and risk characterization.95
Quantitative microbial risk assessment (QMRA)
QMRA ultimately aims to estimate the potential human health risks associated with the presence of pathogenic microorganisms in a water or food source. The health risks are calculated based on what is known or what can be inferred from the concentrations of a certain pathogen in a specific source and the infectivity of that microorganism in humans. Quantitative microbial risk assessment thus differs from epidemiological studies which aim to identify the human health risks by measuring the actual levels of disease in a population.96 Several studies have used water quality information to quantify the microbial health risks associated with the use of water from rainwater catchment systems including for the consumption of untreated water via drinking or spraying with a hose,3,97 aerosol ingestion or inhalation via hosing, showering, or flushing toilets76,97 and the ingestion of untreated harvested rainwater while consuming raw vegetables irrigated with rainwater.98 A 10−4 annual probability of infection or 10−6 disability adjusted life year (DALY) per person per year (pppy) are often used as a tolerable risk metric for drinking water, with risks in excess of this value considered as evidence for the need to explore risk mitigation options.99 For the studies described below, not all risks were computed on an annual basis and therefore are challenging to compare directly to a 10−4 annual infection or 10−6 pppy risk or other available benchmarks for recreational and non-potable uses of water. Where possible, such comparisons are made via application of standard formulas for risk annualization.95
Ahmed and colleagues97 assessed the health risks associated with Salmonella spp., G. lamblia and L. pneumophila concentrations detected in untreated harvested rainwater in SEQ, Australia. For each organism, a set of exposure scenarios were identified. The concentrations of these organisms were measured in 214 roof-harvested rainwater samples collected from 82 tanks using qPCR. A rainwater use survey determined that 65 and 35% of households used rainwater for either (i) outdoor use, including gardening and car washing, or (ii) indoor use, including drinking, showering, and kitchen use, respectively.
It was assumed that all households with rainwater tanks used water for hosing, but only the percentage designated as potable would use the water for showering. Using infection dose-response models, the risk of infection was calculated for each pathogen. The risk of infection from Salmonella spp., G. lamblia, and L. pneumophila associated with the use of rainwater for showering and garden hosing was calculated to be below the threshold value of 10−4 pppy. However, the risk of infection from ingesting Salmonella spp. (9.8 × 100 to 5.4 × 101) and G. lamblia (1.0 × 101 to 6.5 × 101) via drinking water exceeded this value.
Lim and Jiang98 also assessed the potential human health risks associated with Giardia lamblia and Salmonella spp. in harvested rainwater, but more specifically they focused on the risk associated with irrigating homegrown vegetables (cucumbers, lettuce, and tomatoes) with untreated harvested rainwater and then ingesting the vegetables. The volume of water retained on the surface of the vegetables was estimated by weighing the vegetables, submerging the vegetables in the rainwater and then weighing the vegetables again, with the mass difference indicating the retained volume of water on the surface of the produce. The dose of the pathogens consumed by an individual was subsequently estimated by considering the produce consumption or intake rate, body weight and the volume of harvested rainwater retained on the surface of the various crops. Overall, the results indicated that consuming raw lettuce posed the greatest risk of infection for both Giardia lamblia and Salmonella spp., followed by tomatoes and cucumbers. Mean annual giardiasis risks for cucumber consumption were 5.53 × 10−4 (95th percentile 7.58 × 10−4) compared to lettuce (5.49 × 10−3 (95th percentile 6.50 × 10−3) or tomatoes (1.40 × 10−3 (95th percentile 1.87 × 10−3). Mean annual salmonellosis risks ranged from 1.39 × 10−4 (cucumber) 1.09 × 10−3 (lettuce), demonstrating some differences in crop risks. Risk estimates were therefore above a benchmark risk of 10−4 pppy by up to an order of magnitude in some cases, however, the authors mention the possibility of comparing food risks to a benchmark of 10−3 as recommended.95 The authors hypothesized that the higher infection risk (>10−4 pppy) associated with the consumption of lettuce could be due to the higher water retention rate as compared to the retention rates observed for the tomatoes and cucumbers.
Fewtrell and Kay76 examined the microbial risks associated with Campylobacter infection from toilet flushing with rainwater in the UK. The authors used previously published data on Camplylobacter spp. prevalence in rainwater. Monte Carlo analysis was conducted to estimate human health risk, which was quantified in terms of disability adjusted life years (DALYs). The authors assumed that overall, 30% of Campylobacter infections result in illness, while the severity of infections and the duration of illness were also considered. The results indicated that the risk of acquiring campylobacteriosis from using untreated harvested rainwater for toilet flushing was below a risk benchmark of 10−6 DALYs pppy.
Hamilton and colleagues33 further investigated the human health risks associated with the use and consumption of untreated harvested rainwater and specifically focused on the health risks posed by L. pneumophila and the Mycobacterium avium complex (MAC). The exposure scenarios considered for L. pneumophila included inhalation of water droplets while (i) showering, (ii) topping-up the swimming pool, (iii) hosing the garden, (iv) car washing and (v) toilet flushing. These exposure routes were also considered for MAC inhalation, in combination with exposure due to ingestion via (i) drinking, (ii) eating irrigated produce iii) showering and iv) bathing (specific to children). The authors also assessed risks separately for immunocompromised individuals and children as compared to the general population. The results from this study indicated that the median total annual risk due to the inhalation of L. pneumophila was 5 to 6 orders of a magnitude higher than the median total annual risk recorded for the inhalation of MAC. In addition, the 95% confidence interval for the L. pneumophila total risk exceeded the recommended benchmark of 10−4 pppy for infection due to combined uses of water containing pathogen. These risks were driven by showering and recreational exposures for L. pneumophila. Overall, the authors concluded that both inhalation and ingestion of untreated harvested rainwater during drinking, showering and hosing, poses the greatest health risks to the end-users, while car washing and laundry washing may be the most appropriate uses of untreated rainwater for a general population. In contrast, the consumption of lettuce irrigated with untreated harvested rainwater and toilet flushing may be safe for the general population, however, these uses may pose significant health threats to immunocompromised individuals. Findings from this study contradict Lim and Jiang98 study in terms of risk associated with consumption of produce. This is due to the fact that risk assessment undertaken by Lim and Jiang is based on Salmonella spp. and Giardia spp., whereas, Hamilton and colleagues33 estimated risks based on opportunistic pathogens with different dose-response outcomes.
In a risk assessment for splash parks that use rainwater, De Man and colleagues100 used L. pneumophila as a target pathogen to quantify the risk of infection for exposure due to inhalation and C. jejuni for ingestion. Pathogen concentration data were extracted from the literature. The exposure duration was based on an observational study at two splash parks in urban centers, which observed 257 children and 347 adults within 2 m of a water spray. The mean risk/3.5 min exposure duration was 9.3 × 10−5 for children and 1.1 × 10−4 for adults. The authors noted that the duration could be much longer in a recreational water park environment, up to 0.5 h or possibly up to 2 h. Therefore, for a 2 h exposure, the risk would be 2.8 × 10−3. This study showed that splash parks that use rainwater can be associated with a non-trivial infection risk. The results of the sensitivity analysis indicated that the volume of inhalable water spray was the most important input parameter for determining Legionella risk.
In contrast to using untreated RHRW, Schoen and colleagues101 modeled the annual probability of illness from the inhalation of treated rainwater (sand filtration and UV disinfection) while showering, for Campylobacter jejuni, Salmonella enterica, Cryptosporidium spp. Giardia spp. E. coli O157:H7, norovirus and L. pneumophila. This risk assessment was performed in order to compare decentralized community water services to conventional centralized services. The results indicated that the highest annual risk of infection associated with showering in treated rainwater was linked to the inhalation of Cryptosporidium (1.4 × 10−3), followed by E. coli O157:H7 (1.3 × 10−4), Giardia (4.4 × 10−6), L. pneumophila (2.7 × 10−7), Campylobacter (7.9 × 10−8) and Salmonella (7.7 × 10−9), while the health risk posed by the inhalation of norovirus was negligible. In this case, only the risks for Cryptosporidium and E. coli were above a risk benchmark of 10−4 pppy. Even though some risks were below benchmark values, based on these risk values, the authors highlighted that any failure in the treatment system used to disinfect the RHRW could lead to increased human health risks associated with using the water for showering purposes. These increased risks may be significant in terms of Cryptosporidium and E. coli O157:H7 exposure, as they already pose a much higher risk of infection as compared to the other organisms analyzed. Furthermore, the authors noted that the health risks associated with the use of treated rainwater remains uncertain and may change depending on the sites used to collect the rainwater and it may also depend on treatment efficiencies.
Of the six QMRA models discussed here, 5/6 identified risks above commonly used benchmarks for acceptable risk (10−4 probability of infection or 10−6 DALY pppy) for their respective scenarios. As pointed out by Lim and Jiang98, these benchmarks may be unnecessarily stringent for rainwater, and relative risk comparisons with other water sources can yield more meaningful results in some cases.
There are several limitations associated with QMRA for rainwater that can affect the interpretation of computed risk values. Risk assessment using QMRA often relies on static models to predict health risks based on a single exposure event to a particular pathogen. These models do not consider properties associated with disease transmission such as population dynamics, immunity and secondary transmission.96 In addition, although QMRA provides useful risk estimates, these estimates hold more value when used to guide research and development towards contamination prevention and remediation strategies, rather than identifying risk for a certain set of environmental conditions and input parameters at a specific time and/or location.102
Therefore, it may be more useful to report risk estimates obtained by QMRA, as “tolerable pathogen concentrations in water” or “log removal required” for a water source to be deemed safe, as this could guide the practical implementation and improvement of treatment systems.102 This may be done by calculating disability adjusted life year (DALY) scores and comparing these scores to the WHO guidelines.103 However, numeric DALY values for computing the burden of disease from a given pathogen are regionally specific. The aggregation of additional information for computing DALY values such as the incidence at a severity level, total incidence, odds of severity, and duration of illness for various populations would be valuable in order to make such comparisons. Such an analysis has been carried out to support QMRA efforts previously.98 Furthermore, QMRA relies on specific input parameters, and therefore, the results are often applicable only to a particular scenario. Thus, the reporting of risk estimates as log reduction benchmarks or tolerable pathogen concentrations per water source, is further warranted. In addition, it should be noted that dose-response studies are mostly conducted on healthy individuals and therefore may not reflect the true response of the general population or the more susceptible portion of the population such as children, immunocompromised individuals and the elderly.96 It is also difficult to extrapolate dose-response models from one strain to obtain a general model for a pathogen, as virulence may differ between strains of the same species.96
Future research should focus on developing dose-response models for opportunistic and pathogenic microorganisms associated with RHRW for which there are no dose-response models available (such as Aeromonas spp., Clostridium perfringens, Streptococcus spp., Pseudomonas syringae and Klebsiella pneumoniae). Risk analysis can be used to estimate the amount of treatment that would be needed to meet different risk targets; reporting this information would be useful to risk managers.
Disinfection and maintenance of rainwater
Various treatment technologies have been employed to reduce the level of chemical and microbial contaminants in RHRW to within drinking water standards (Table 3). The majority of treatment strategies have focused on reducing the entry of contaminants into rainwater harvesting (RWH) tanks, the utilization of chemical disinfectants, filtration techniques, or ultra-violet (UV) and heat treatment (Table 3). In order to minimize the entry of microbial contaminants into a RWH tank, rainwater pre-treatment systems (i.e., implementation of gutter screens or first flush diverters), have been investigated.36,104 Gutter screens prevent the entry of leaves and large organic debris into the conveyance system, while a first flush diverter collects the first amount of run-off water, which is considered to have the highest concentration of contaminants.105 Although it has been reported that the use of first flush diverters and other pre-treatment techniques, primarily improve the physico-chemical quality of RHRW,104 improvements in the microbial quality of RHRW have also been reported. While comparing the quality of first flush water to RHRW collected following the first flush, Mendez and colleagues36 reported total coliform log reductions ranging from 0.10 to 0.37 depending on roofing material type. Similarly, Lee and colleagues37 reported total coliform log reductions ranging from 1.04 to 1.84 using a first flush diverter. Moreover, diverting the first consortium of contaminants may effectively reduce the turbidity of the rainwater,106 which is beneficial as increased water turbidity may influence secondary treatment strategies by shielding microorganisms from UV-radiation, react with chemical disinfectants such as chlorine and lead to clogging of filtration systems.107 It is thus recommended that first flush diverters should be employed for the initial pre-treatment of RHRW.106 This is beneficial as increased water turbidity may shield microorganisms from UV-radiation, react with chemical disinfectants such as chlorine and lead to clogging of filtration systems.107
Chlorination is considered an effective, inexpensive and simple way of treating household water supplies and reduces the incidence of diarrheal disease in developing countries by 20–48%.107 Chlorine and chlorine-based compounds destroy microorganisms by binding to target sites on the cell surface, which causes the release of vital cellular components and in turn terminates membrane-associated functions and metabolism within the cell.107,108 However, certain microorganisms have been shown to display increased resistance to chlorination.109 Although chlorine treatment may prevent microbial re-growth if sufficient residual chlorine is available,15,110 the effectiveness of any disinfectant is generally short lived and will only act on the water at the time of dosing as the entry of fresh RHRW run-off into the tank will dilute the residual chlorine concentration.15,109,110 While investigating rainwater quality in Greece, Sazakli and colleagues26 reported that chlorination at 0.4–0.5 mg/L for at least 15 min lead to satisfactory disinfection of rainwater. However, as chlorine may react with organic material inside the tank (organic material may settle and accumulate at the bottom of the tank) and form undesirable by-products, it has been recommended that rainwater is dosed with chlorine after it has been removed from the tank.111
Various filtration techniques have also been assessed for the treatment of RHRW (Table 3) with the filtration mechanisms ranging from a physical removal approach (e.g., microfiltration, ceramic filtration) to biological treatment processes (e.g., slow-sand filtration).112,113 The effectiveness of physical filtration systems relies on its ability to remove microorganisms based on size. In contrast, slow-sand or bio-sand filtration techniques utilize the increased surface area of the filter to enable biofilm formation, which acts as a biological filter by removing contaminating microorganisms. While assessing point-of-use household drinking water filtration techniques, Sobsey and colleagues113 reported that ceramic filtration yielded baseline log reduction values of 2, 0.5, and 4, for the removal of bacteria, viruses and protozoa, respectively, while bio-sand filtration yielded baseline log reduction values of 1, 0.5, and 2, respectively. A major advantage of using filtration is that it can remove both microbial and chemical contaminants from RHRW. However, disadvantages include prolonged treatment time and in certain systems, such as slow-sand filtration systems, components may need to be replaced in order for the system to remain efficient.15,107
Solar disinfection (SODIS) utilizes the synergistic effects of light (UV) and mild heat to inactivate microbial contaminants.114 In its simplest form, transparent bottles are filled with the water to be treated and are exposed to direct sunlight for 6–48 h.114 Although SODIS has successfully been utilized to reduce bacterial, fungal, protozoan and viral contaminants in water, certain organisms including fecal coliforms and gram-positive endospore formers, display slower inactivation rates and require longer exposure times.115 Moreover, as organisms may initiate photo reactivation mechanisms (repair of DNA following UV damage), it has been recommended that SODIS treated water be used within 24 h to avoid post-treatment re-growth.114
While investigating SODIS using a solar cooker for the treatment of RHRW, Strauss and colleagues116 reported that SODIS was able to reduce E. coli (>2 log reduction) and heterotrophic bacteria (>6 log reduction) to within drinking water standards. However, utilizing the ethidium monoazide bromide (EMA)-qPCR technique, the authors reported that intact Legionella spp. and Pseudomonas spp. were still detected in the samples following SODIS treatment (Table 3). The major disadvantages of using conventional SODIS for the treatment of RHRW however, is the limited volumes of water that can be treated and the technique’s inefficiency under poor weather conditions.113,114 Additionally, conflicting conclusions regarding the influence of turbidity on SODIS efficiency have been reported.117,118
In order to increase the efficiency of SODIS, various enhancement technologies have thus been assessed, including the use of flow reactors and larger reactor tubes (increase treatment volume), solar mirrors (concentrate UV) and the addition of heterogeneous photocatalysts and chemical additives (increase production of reactive oxygen species).114 While investigating the efficiency of filtration combined with UV disinfection (UV lamp) for the treatment of RHRW, Kim and colleagues119 reported a 0.3 log reduction in total coliforms under low intensity (IUVA = 5.4 W/m2) UV treatment for 5 min, with the treatment efficiency increasing with treatment time (0.52 log reduction after 60 min). In contrast, Jordan and colleagues11 reported >2 log10 reductions in E. coli and total coliforms using a higher UV treatment intensity (22 W lamp) (Table 3).
In recent years, research has also focused on the development of solar pasteurization (SOPAS) systems that utilize solar energy to treat RHRW.120 Advantages of SOPAS include its ability to treat large volumes of water with the removal of microbial pathogens being independent of turbidity, pH and additional parameters that may influence chemical disinfection and SODIS treatments.121 While investigating the efficiency of SOPAS systems to treat RHRW, Dobrowsky and colleagues122 and Reyneke and colleagues123 reported the reduction in traditional indicator organisms (e.g., E. coli, total coliforms, heterotrophic bacteria) to below the detection limit (<1 CFU/100 mL). However, using the EMA-qPCR technique, Reyneke and colleagues124 showed that intact Legionella spp. (1.4 × 104 GC/mL DNA) were still present in SOPAS treated rainwater (Table 3). Similarly, Strauss and colleagues116 detected intact Pseudomonas spp. (7.31 × 104 GC/mL DNA) at temperatures greater than 90 °C after SOPAS treatment (Table 3). A further disadvantage of SOPAS is that it does not improve the chemical quality of treated water.125
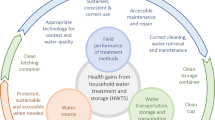
As various advantages and disadvantages are associated with the various treatment methods, depending on the specific application [i.e. drinking, washing, cleaning, supplementation of greywater uses (e.g. toilet flushing, irrigation)], a combination of treatment methods may be required to adequately treat RHRW.112 Based on literature, the use of filtration in combination with another physical or a chemical treatment method is efficient (>3 log reductions of microbial contaminants) and widely employed (Table 3). It is however, important to note that the treatment of RHRW is not the final step in reducing the potential health risks associated with using this water source, as unsafe consumer water handling practices (inadequate and unsanitary storage conditions, specifically if the water is stored for a prolonged period of time) have also been shown to contribute to the re-contamination of treated water.107 Regarding the control of opportunistic pathogens such as Legionella spp. it is recommended to design a building water safety plan as recommended by the WHO, Centers for Disease Control and Prevention (CDC) and American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE) guidelines. This can involve reducing water age, maintaining a disinfectant residual, and/or flushing distal taps where appropriate to reduce risks.
Quantitative knowledge regarding rainwater system maintenance practices and relationships to water quality in various contexts remains a challenging parameter to address in risk assessments. Although treatment schemes to purify RHRW do exist, they can sometimes be complicated, and the most effective means of keeping RHRW clean is prevention of contamination through regular maintenance.126 However, as individual owners are often solely responsible for RHRW quality, the implementation of maintenance and treatment strategies is highly variable and can be burdensome. An early study by Lye127 reported 16/30 of Kentucky households never disinfected their water prior to drinking or did not use a filter (21/30), while 22/30 did not annually clean their systems or divert the first flush (2/30). Stump and colleagues128 surveyed 36 Texas households who were members of the Texas Rainwater Catchment Association, finding that 81% of households maintained their filters every 6 months, but 19% only did so every 6–12 months. A study of Bermuda rainwater tanks found that cleaning the tank the year before sampling resulted in a lower concentration of E. coli, however of the 102 households surveyed, 48% disinfected their tanks and only 40% did so on a regular basis.48 In a study in Queensland, Australia, of 121 survey respondents, 27% of potable and non-potable users treated their RHRW, and only 50% of potable users treated their RHRW.33,46
In a follow-up study of a subset of 24 tanks (23 responses were gathered) from the original 121, 46% had a first-flush diverter and 38% treated their water (all using filtration).33 In a survey of the American Rainwater Catchment Systems Association (representing 2,700 RHRW systems across the US), 51 and 54% of individual and business users had a first flush system installed, respectively.129 Users commonly had installed a roof washer (filter upstream of the cistern) (67 and 73% for individual and business users, respectively).129 It is also likely that RHRW quality testing is infrequent; Thomas and colleagues129 noted that 21% of individual respondents and 37% of business respondents did not conduct any microbial water quality testing, while Stump and colleagues128 noted that 64% of 36 Texas households had never had their RHRW quality tested.
Due to presumed low maintenance and treatment rates among cistern owners, more information is needed to determine which applications of untreated rainwater drive risks, and which risk reduction practices can most effectively manage water quality. When considering uses besides drinking, irrigation of urban community gardens with RHRW is of particular concern due to potential exposure to contaminants.
Conclusions
-
E. coli has been consistently found in extensive monitoring of harvested rainwater supplies, failing to meet most criteria for suitable drinking water quality.
-
While FIB have been consistently found in RHRW, fewer data are available regarding enteric and opportunistic pathogens and protozoas.
-
Sources of fecal material in RHRW have been identified using microbial source tracking approaches. However, more specific information regarding sources of pathogenic microorganisms in rainwater tanks would be helpful for managing risks.
-
Molecular biology methods such as qPCR have been commonly used to quantify microorganisms in rainwater tanks. While culture-based approaches may underestimate the presence of various microorganisms, molecular approaches generally do not provide information on cell viability. Alternative approaches such as PMA- or EMA- qPCR may have caveats as they are based on cell membrane integrity. Therefore, additional analysis is needed to assess viability and infectivity for evaluating risks.
-
Approaches for assessing the microbial community of rainwater tanks such as sequencing have identified pathogenic genera. However, the extent to which specific non-pathogenic microbial constituents can provide benefits or incur risks to human health has not been determined.
-
The findings from epidemiological studies indicate that RHRW can provide a decreased risk of diarrheal illness compared to consuming alternative or unimproved water sources. However, when risks are evaluated for RHRW relevant to other sources (such as “improved” water sources), this comparison is contingent upon the quality and context of the alternative water source, and does not provide information on absolute risk and its relationship to risk acceptability benchmarks. It is noted here that in epidemiologic and outbreak investigation studies, examining the relationship between exposures to waterborne pathogens and disease can be complicated by multiple factors.
-
Quantitative microbial risk assessments can provide useful frameworks for quantifying and prioritizing these factors. Additional information is needed to address gaps in dose response parameters for assessing a complete suite of pathogenic risks for a given exposure scenario. The assessment of these risks is specific to the intended water usage purpose, frequency, and duration; the concentration of pathogen(s) present for a particular season/time period; rainwater harvesting system maintenance and treatment practices; system performance; and the (potentially susceptible) population at risk.
-
Risk mitigation for pathogens (and other water quality hazards) is possible using effective engineering designs and available treatment methods such as solar disinfection, chemical disinfection, filtration, and first-flush diversion. Small-scale usage of treatment techniques has been demonstrated to be effective under certain circumstances, however long-term performance and optimal use of these techniques given various engineering designs and materials, human usage and maintenance schemes, and variable in-field environmental conditions remains a gap for further inquiry.
References
Ahmed, W., Goonetilleke, A. & Gardner, T. Implications of fecal indicator bacteria for the microbiological assessment of roof-harvested rainwater quality in Southeast Queensland, Australia. Can. J. Microbiol. 56, 471–479 (2010).
Eberhart-Phillips, J. et al. Campylobacteriosis in New Zealand: results of a case-control study. J. Epidemiol. Community Health 51, 686–691 (1997).
Lye, D. J. Health risks associated with consumption of untreated water from household roof catchment systems. J. Am. Water Resour. Assoc. 38, 1301–1306 (2002).
Simmons, G. et al. Legionnaires’ disease outbreak: a water blaster and roof-collected rainwater systems. Water Res. 42, 1449–1458 (2008).
Ahmed, W. & Toze, S. in Rainwater Tank Systems for Urban Water Supply. Microbiological Quality and Associated Health Risks with the Use of Roof-Captured Rainwater. (eds Sharma, D. Begbie, T. Gardner) 229–249 (International Water Association Publishing, London, 2014).
van Dijk, A. I. J. M. et al. The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resour. Res. 49, 1040–1057 (2013).
ACT Government. Think Water, Act Water Strategy: Strategy for sustainable water resource management in the ACT (2004). http://www.thinkwater.act.gov.au/.
Walton, C. & Holmes, K. How much water efficiency does $321 million buy? In Proceedings of the 5th IWA Specialist Conference ‘Efficient 2009’ on Efficient Use and Management of Urban Water. (International Water Association (IWA) and Australian Water Association, Sydney, 2009).
DIP. Water saving targets. For councils,.plumbers, builders and developers. A guide to the Queensland Development Code Part MP 4.2, Department of Infrastructure and Planning (2008).
Farreny, R. et al. Roof selection for rainwater harvesting: quantity and quality assessments in Spain. Water Res. 45, 3245–3254 (2011).
Jordan, F. S., Seamn, R., Riley, J. J. & Yoklic, M. R. Effective removal of microbial contamination from harvested rainwater using a simple point of use filtration and UV-disinfection device. Urban Water J. 5, 209–218 (2008).
Vialle, C. et al. Monitoring of water quality from roof runoff: Interpretation using multivariate analysis. Water Res. 45, 3765–3775 (2011).
Abbasi, T. & Abbasi, S. Sources of pollution in rooftop rainwater harvesting systems and their control. Crit. Rev. Environ. Sci. Technol. 41, 2097–2167 (2011).
Ahmed, W., Gardner, T. & Toze, S. Microbiological quality of roof-harvested rainwater and health risks: a review. J. Environ. Qual. 40, 13–21 (2011).
De Kwaadsteniet, M., Dobrowsky, P. H., van Deventer, A. & Cloete, T. E. Domestic rainwater harvesting: microbial and chemical water quality and point-of-use treatment systems. Water Air Soil Pollut. 224, 1629 (2013).
Berndtsson, J. C. Green roof performance towards management of runoff water quantity and quality: a review. Ecol. Eng. 36, 351–360 (2010).
Chapman, H. C., Cartwright, T., Huston, R. & O’Toole, J. Water Quality and Health Risks from Urban Rainwater Tanks. (Cooperative Research Centre for Water Quality and Treatment, Salisbury SA, 2008).
Clark, S. E. et al. Roofing materials’ contributions to storm-water runoff pollution. J. Irrig. Drain E ASCE. 134, 638–645 (2008).
Gobel, P., Dierkes, C. & Coldewey, W. G. Storm water runoff concentration matrix for urban areas. J. Contam. Hydrol. 91, 26–42 (2007).
Meera, V. & Ahammed, M. Water quality of rooftop rainwater harvesting systems: a review. Water Supply. 55, 257–268 (2006).
Lye, D. J. Microbiology of rainwater cistern systems: a review. J. Environ. Sci. Health A. 27, 2123–2166 (1992).
Hatt, B., Deletic, A. & Fletcher, T. Integrated stormwater treatment and re-use systems: inventory of Astralian practice. CRC for Catchment Hydrology (2004).
Handia, L. Comparative study of rainwater quality in urban Zambia. J. Water Supply Res. T-Aqua. 54, 55–64 (2005).
Melidis, P., Akratos, C. S., Tsihrintzis, V. A. & Trikilidou, E. Characterization of rain and roof drainage water quality in Xanthi, Greece. Environ. Monit. Assess. 127, 15–27 (2007).
Peters, A. J., Weidner, K. L. & Howley, C. L. The chemical water quality in roof-harvested water cisterns in Bermuda. J. Water Supply Res. T-Aqua. 57, 153–163 (2008).
Sazakli, E., Alexopoulos, A. & Leotsinidis, M. Rainwater harvesting, quality assessment and utilization in Kefalonia Island, Greece. Water Res. 41, 2039–2047 (2007).
Thomas, T. Domestic water supply using rainwater harvesting. Build. Res. Inf. 26, 94–101 (1998).
Brodie, E. L. et al. Urban aerosols harbor diverse and dynamic bacterial populations. Proc. Natl Acad. Sci. USA 104, 299–304 (2007).
Evans, C. A., Coombes, P. J. & Dunstan, R. H. Wind, rain and bacteria: the effect of weather on the microbial composition of roof-harvested rainwater. Water Res. 40, 37–44 (2006).
Huston, R., Chan, Y. C., Gardner, T., Shaw, G. & Chapman, H. Characterization of atmospheric deposition as a source of contaminants in urban rainwater tanks. Water Res. 43, 1630–1640 (2009).
Lye, D. J. Rooftop runoff as a source of contamination: a review. Sci. Total Environ. 407, 5429–5434 (2009).
Ahmed, W., Hamilton, K., Gyawali, P., Toze, S. & Haas, C. Evidence of avian and possum fecal contamination in rainwater tanks as determined by microbial source tracking approaches. Appl. Environ. Microbiol. 82, 4379–4386 (2016).
Hamilton, K. A. et al. Seasonal assessment of opportunistic premise plumbing pathogens in roof-harvested rainwater tanks. Environ. Sci. Technol. 51, 1742–1753 (2017).
Wang, H. et al. Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, Mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environ. Sci. Technol. 46, 11566–11574 (2012).
Rhoads, W. J., Pruden, A. & Edwards, M. A. Survey of green building water systems reveals elevated water age and water quality concerns. Environ. Sci. 2, 164–173 (2016).
Mendez, C. B. et al. The effect of roofing material on the quality of harvested rainwater. Water Res. 45, 2049–2059 (2011).
Lee, J. Y., Bak, G. & Han, M. Quality of roof-harvested rainwater—comparison of different roofing materials. Environ. Pollut. 162, 422–429 (2012).
Forster, J. Variability of roof runoff quality. Water Sci. Technol. 39, 137–144 (1999).
Schriewer, A., Horn, H. & Helmreich, B. Time focused measurements of roof runoff quality. Corros. Sci. 50, 384–391 (2008).
Yaziz, M. I., Gunting, H., Sapari, N. & Ghazali, A. W. Variations in rainwater quality from roof catchments. Water Res. 23, 761–765 (1989).
Ahmed, W., Gyawali, P. & Toze, S. Quantitative PCR measurements of Escherichia coli including shiga toxin-producing E. coli (STEC) in animal feces and environmental waters. Environ. Sci. Technol. 49, 3084–3090 (2015).
Leong, J. Y. C., Chong, M. N., Poh, P. E., Hermawan, A. & Talei, A. Longitudinal assessment of rainwater quality under tropical climatic conditions in enabling effective rainwater harvesting and reuse schemes. J. Clean. Prod. 143, 64–75 (2017).
Ahmed, W., Richardson, K., Sidhu, J. P. S. & Toze, S. Escherichia coli and Enterococcus spp. in rainwater tank samples: comparison of culture-based methods and 23S rRNA gene quantitative PCR assays. Environ. Sci. Technol. 46, 11370–11376 (2012).
Islam, M. A., Sakakibara, H., Karim, M. R., Sekine, M. & Mahmud, Z. H. Bacteriological assessment of drinking water supply options in coastal areas of Bangladesh. J. Water Health 9, 415–428 (2011).
Ahmed, W., Brandes, H., Gyawali, P., Sidhu, J. P. S. & Toze, S. Opportunistic pathogens in roof-captured rainwater samples, determined using quantitative PCR. Water Res. 53, 361–369 (2014).
Hamilton, K. A. et al. Public health implications of Acanthamoeba and multiple potential opportunistic pathogens in roof-harvested rainwater tanks. Environ. Res. 150, 320–327 (2016).
Ahmed, W., Hamilton, K. A., Toze, S. & Haas, C. N. Seasonal abundance of fecal indicators and opportunistic pathogens in roof-harvested rainwater tanks. Open Health Data. 5, 1 (2018).
Levesque, B. et al. Assessment of microbiological quality of drinking water from household tanks in Bermuda. Can. J. Microbiol. 54, 495–500 (2008).
Ahmed, W., Hodgers, L., Sidhu, J. P. S. & Toze, S. Fecal indicators and zoonotic in household drinking water taps fed from rainwater tanks in Southeast Queensland, Australia. Appl. Environ. Microbiol. 78, 219–226 (2012b).
Abbott, S. E., Douwes, J. & Caughley, B. P. A survey of the microbiological quality of roof-collected rainwater of private dwellings in New Zealand. J. Environ. Health 29, 6–16 (2006).
Dobrowsky, P. H. et al. Prevalence of virulence genes associated with pathogenic Escherichia coli strains isolated from domestically harvested rainwater during low- and high-rainfall periods. Appl. Environ. Microbiol. 80, 1633–1638 (2013).
Kaushik, R., Balasubramanian, R. & de la Cruz, A. A. Influence of air quality on the composition of microbial pathogens in fresh rainwater. Appl. Environ. Microbiol. 78, 2813–2818 (2012).
He, J. & Balasubramanian, R. Rain-aerosol coupling in the tropical atmosphere of Southeast Asia: distribution and scavenging ratios of major ionic species. J. Atmos. Chem. 60, 205–220 (2008).
Kaushik, R., Balasubramanian, R. & Dunstan, H. Microbial quality and phylogenetic diversity of fresh rainwater and tropical freshwater reservoir. PLoS One 9, e100737 (2014).
Schoen, M. E., Ashbolt, N. J., Jahne, M. A. & Garland, J. Risk-based enteric pathogen reduction targets for non-potable and direct potable use of roof runoff, stormwater, and greywater. Microb. Risk Anal. 5, 32–43 (2017).
Daoud, A. K., Swaileh, K. M., Hussein, R. M. & Matani, M. Quality assessment of roof-harvested rainwater in the West Bank, Palestinian Authority. J. Water Health 9, 525–533 (2011).
Ahmed, W. et al. Occurrence of intestinal and extraintestinal virulence genes in Escherichia coli isolates from rainwater tanks in Southeast Queensland, Australia. Appl. Environ. Microbiol. 77, 7394–7400 (2011).
Crabtree, K. D., Ruskin, R. H., Shaw, S. B. & Rose, J. B. The detection of Cryptosporidium oocysts and Giardia cysts in cistern water in the U.S. Virgin Islands. Water Res. 30, 208–216 (1996).
Simmons, G., Hope, V., Lewis, G., Whitmore, J. & Gao, W. Contamination of potable roof-collected rainwater in Auckland, New Zealand. Water Res. 35, 1518–1524 (2001).
Albrechtsen, H.-J. Microbiological investigations of rainwater and graywater collected for toilet flushing. Water Sci. Technol. 46, 311–316 (2002).
Ahmed, W., Huygens, F., Goonetilleke, A. & Gardner, T. Real-time PCR detection of pathogenic microorganisms in roof-harvested rainwater in Southeast Queensland, Australia. Appl. Environ. Microbiol. 74, 5490–5496 (2008).
Waso, M., Ndlovu, T., Dobrowsky, P. H., Khan, S. & Khan, W. Presence of microbial and chemical source tracking markers in roof-harvested rainwater and catchment systems for the detection of fecal contamination. Environ. Sci. Pollut. Res. 23, 16987–17001 (2016).
Al-Bahry, S. N., Elshafie, A. E., Victor, R., Mahmoud, I. Y. & Al-Hinai, J. A. Opportunistic pathogens relative to physicochemical factors in water storage tanks. J. Water Health 9, 382–393 (2011).
Lau, H. Y. & Ashbolt, N. J. The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. J. Appl. Microbiol. 107, 368–378 (2009).
Falkinham, J. O. III, Norton, C. D. & LeChevallier, M. W. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67, 1225–1231 (2001).
Långmark, J., Storey, M. V., Ashbolt, N. J. & Stenström, T. A. Accumulation and fate of microorganisms and microspheres in biofilms formed in a pilot-scale water distribution system. Appl. Environ. Microbiol. 71, 706–712 (2005).
Lin, W., Yu, Z., Chen, X., Liu, R. & Zhang, H. Molecular characterization of natural biofilms from household taps with different materials: PVC, stainless steel, and cast iron in drinking water distribution system. Appl. Microbiol. Biotechnol. 97, 8393–8401 (2012).
Evans, C. A., Coombes, P. A., Dunstan, R. H. & Harrison, T. Extensive bacterial diversity indicates the potential operation of a dynamic micro-ecology within domestic rainwater storage systems. Sci. Total Environ. 407, 5206–5215 (2009).
Wang, J. et al. Environmental bio-monitoring with high-throughput sequencing. Brief. Bioinform. 14, 575–588 (2013).
Chidamba, L. & Korsten, L. Pyrosequencing analysis of roof-harvested rainwater and river water used for domestic purpose in Luthengele village in the Eastern Cape province of South Africa. Environ. Monit. Assess. 187, 41 (2015).
Ahmed, W. et al. Amplicon-based taxonomic characterization of bacteria in urban and peri-urban roof-harvested rainwater stored in tanks. Sci. Total Environ. 576, 326–334 (2017).
Jongman, M. & Korsten, L. Microbial quality and suitability of roof-harvested rainwater in rural villages for crop irrigation and domestic use. J. Water Health 14, 961–971 (2016).
Dobrowsky, P. H. et al. Quality assessment and primary uses of harvested rainwater in Kleinmond, South Africa. Water SA 40, 401–406 (2014b).
Harwood, V. J., Staley, C., Badgley, B. D., Borges, K. & Korajkic, A. Microbial source tracking markers for detection of faecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 38, 1–40 (2014).
Waso, M., Khan, S. & Khan, W. Microbial source tracking markers associated with domestic rainwater harvesting systems: correlation to indicator organisms. Environ. Res. 161, 446–455 (2018).
Fewtrell, L. & Kay, D. Quantitative microbial risk assessment with respect to Campylobacter spp. in toilets flushed with harvested rainwater. Water Environ. J. 21, 275–280 (2007).
Murrell, W. G. & Stewart, B. J. Botulism in New South Wales, 1980-1981. Med. J. Aust. 1, 13–17 (1983).
Koplan, J. D., Deer, R. D., Swanston, W. H. & Tota, B. Contaminated roof-collected rainwater as a possible cause of an outbreak of salmonellosis. J. Hyg. 81, 303–309 (1978).
Heyworth, J. S., Glonek, G., Maynard, E. J., Baghurst, P. A. & Finlay-Jones, J. Consumption of untreated tank rainwater and gastroenteritis among young children in South Australia. Int. J. Epidemiol. 35, 1051–1058 (2006).
Rodrigo, S., Ledar, K. & Sinclair, M. Quality of stored rainwater used for drinking in metropolitan South Australia. The Cooperative Research Centre for Water Quality and Treatment. Report no. 84 (2009).
Few, R., Lake, I., Hunter, P. R., Tran, P. G. & Thien, V. T. Seasonal hazards and health risks in lower-income countries: field testing a multi-disciplinary approach. Environ. Health 8, S16 (2009).
Garrett, V. et al. Diarrhoea prevention in a high-risk rural Kenyan population through point-of-use chlorination, safe water storage, sanitation, and rainwater harvesting. Epidemiol. Infection 136, 463–1471 (2008).
Kelly-Hope, L. A. et al. Geographical distribution and risk factors associated with enteric diseases in Vietnam. Am. J. Trop. Med. Hyg. 76, 706–712 (2007).
Marcynuk, P. B. et al. Comparison of the burden of diarrhoeal illness among individuals with and without household cisterns in northeast Brazil. BMC Infect. Dis. 13, 65 (2013).
Rodrigo, S., Sinclair, M., Forbes, A., Cunliffe, D. & Leder, K. Drinking rainwater: a double-blinded, randomized controlled study of water treatment filters and gastroenteritis incidence. Am. J. Pub. Health 101, 842–847 (2011).
El Saadi, O. E., Esterman, A. J., Cameron, S. & Roder, D. M. Murray River water, raised cyanobacterial cell counts, and gastrointestinal and dermatological symptoms. Med. J. Aust. 162, 122–125 (1995).
Dean, J. & Hunter, P. R. Risk of gastrointestinal illness associated with the consumption of rainwater: a systematic review. Environ. Sci. Technol. 46, 2501–2507 (2012).
Franklin, L. J. et al. An outbreak of Salmonella Typhimurium 9 at a school camp linked to contamination of rainwater tanks. Epidemiol. Infect. 137, 434–440 (2009).
Kuroki, T. et al. An outbreak of waterborne cryptosporidiosis in Kanagawa, Japan. Kansenshogaku zasshi. J. Jpn Assoc. Infect. Dis. 70, 132–140 (1996).
Mpogui, A. Consumption of rainwater and diarrheal disease in children under five in the Dominican Republic from 2002 to 2007. 2012, Georgia State University Institute of Public Health. p. 54 (2012).
Dean, J. M. E., Deare, F., Kydd, K., Ward-Robinson, J. & Hunter, P. R. Rainwater harvesting in rural Trinidad; a cross sectional, observational study. J. Water Sanit. Hyg. Dev. 2, 241–249 (2012).
Fonseca, J. E. et al. Reducing occurrence of Giardia duodenalis in children living in semiarid regions: impact of a large scale rainwater harvesting initiative. PLoS Negl. Trop. Dis. 8, e2943 (2014).
Pham-Duc, P. et al. Diarrhoeal diseases among adult population in an agricultural community Hanam province, Vietnam, with high wastewater and excreta re-use. BMC Public Health 14, 978 (2014).
Emont, J. P., Ko, Al, Homasi-Paelate, A., Ituaso-Conway, N. & Nilles, E. J. Epidemiological Investigation of a diarrhea outbreak in the South Pacific Island nation of Tuvalu during a severe La Niña–associated drought emergency in 2011. Am. J. Trop. Med. Hyg. Pii, 16–0812 (2017).
Haas, C. N., Rose, J. B. & Gerba, C. P. Quantitative Microbial Risk Assessment. (Wiley, Hoboken, 1999).
Hunter, P. R., Payment, P., Ashbolt, N. & Bartram, J. in (eds Ronchi, E. & Bartram, J.) Assessing Microbiological Safety of Drinking Water: Improving Approaches and Methods. 79–109 (Organisation for Economic Co-operation and Development/World Health Organization, Paris, 2003).
Ahmed, W., Vieritz, A., Goonetilleke, A. & Gardner, T. Health risk from the use of roof-harvested rainwater in Southeast Queensland, Australia, as potable or nonpotable water, determined using quantitative microbial risk assessment. Appl. Environ. Microbiol. 76, 7382–7391 (2010).
Lim, K. & Jiang, S. C. Reevaluation of health risk benchmark for sustainable water practice through risk analysis of rooftop-harvested rainwater. Water Res. 47, 7273–7286 (2013).
Regli, S., Rose, J. B., Haas, C. N. & Gerba, C. Modeling the risk from Giardia and viruses in drinking water. J. Am. Water Works Ass. 83, 68–72 (1991).
De Man, H. et al. Health risk assessment for splash parks that use rainwater as source water. Water Res. 54, 254–261 (2014).
Schoen, M. E., Xue, X., Hawkins, T. R. & Ashbolt, N. J. Comparative human health risk analysis of coastal community water and waste service options. Environ. Sci. Technol. 48, 9728–9736 (2014).
Jiang, S. C., Lim, K., Huang, X., McCarthy, D. & Hamilton, A. J. Human and environmental health risks and benefits associated with use of urban stormwater. WIREs Water 2, 683–699 (2015).
WHO. Guidelines for Drinking-Water Quality. Third Edition. Volume 1: Recommendations. (World Health Organization, Geneva, 2004).
Gikas, G. D. & Tsihrintzis, V. A. Assessment of water quality of first-flush roof runoff and harvested rainwater. J. Hydrol. 466–467, 115–126 (2012).
Sánchez, A. S., Cohim, E. & Kalid, R. A. A review on physicochemical and microbiological contamination of roof-harvested rainwater in urban areas. Sustain. Water Qual. Ecol. 6, 119–137 (2015).
Helmreich, B. & Horn, H. Opportunities in rainwater harvesting. Desalination 248, 118–124 (2009).
World Health Organization (WHO). Managing Water in the Home: Accelerated Health Gains from Improved Water Supply (2002).
Venkobachar, C., Leela, L. & Prabhakara, R. Mechanism of disinfection: effect of chlorine on cell membrane functions. Water Res. 11, 727–729 (1977).
Karikari, A. Y. & Ampofo, J. A. Chlorine treatment effectiveness and physico-chemical and bacteriological characteristics of treated water supplies in distribution networks of Accra-Tema Metropolis, Ghana. Appl. Water Sci. 3, 535–543 (2013).
Sobsey, M. D. Inactivation of health-related microorganisms in water by disinfection processes. Water Sci. Technol. 21, 179–195 (1989).
Gordon, G., Adam, L. & Bubnis, B. Minimizing chlorate ion formation. J. Am. Water Works Assoc. 87, 97–106 (1995).
Li, Z., Boyle, F. & Reynolds, A. Rainwater harvesting and greywater treatment systems for domestic application in Ireland. Desalination 260, 1–8 (2010).
Sobsey, M. D., Stauber, C. E., Casanova, L. M., Brown, J. M. & Elliott, M. A. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 42, 4261–4267 (2008).
McGuigan, K. G. et al. Solar water disinfection (SODIS): a review from bench-top to roof-top. J. Hazard. Mater. 235-236, 29–46 (2012).
Sinton, L. W., Hall, C. H., Lynch, P. A. & Davies-Colley, R. J. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68, 1122–1131 (2002).
Strauss, A., Dobrowsky, P. H., Ndlovu, T., Reyneke, B. & Khan, W. Comparative analysis of solar pasteurization versus solar disinfection for the treatment of harvested rainwater. BMC Microbiol. 16, 289–305 (2016).
Meera, V. & Ahammed, M. M. Solar disinfection for household treatment of roof-harvested rainwater. Water Sci. Technol. 8, 153–160 (2008).
Ubomba‐Jaswa, E., Fernández‐Ibáñez, P., Navntoft, C., Polo‐López, M. I. & McGuigan, K. G. Investigating the microbial inactivation efficiency of a 25L batch solar disinfection (SODIS) reactor enhanced with a compound parabolic collector (CPC) for household use. J. Chem. Technol. Biotechnol. 85, 1028–1037 (2010).
Kim, R., Lee, S. & Kim, J. Application of a metal membrane for rainwater utilization: filtration characteristics and membrane fouling. Desalination 177, 121–132 (2005).
Abraham, J. P., Plourde, B. D. & Minkowycz, W. J. Continuous flow solar thermal pasteurization of drinking water: methods, devices, microbiology, and analysis. Renew. Energ. 81, 795–803 (2015).
Burch, J. D. & Thomas, K. E. Water disinfection for developing countries and potential for solar thermal pasteurization. Sol. Energy 64, 87–97 (1998).
Dobrowsky, P. H., Carstens, M., De Villiers, J., Cloete, T. E. & Khan, W. Efficiency of a closed-coupled solar pasteurization system in treating roof harvested rainwater. Sci. Total Environ. 536, 206–214 (2015).
Reyneke, B., Cloete, T. E., Khan, S. & Khan, W. Rainwater harvesting solar pasteurization treatment systems for the provision of an alternative water source in peri-urban informal settlements. Environ. Sci. 4, 291–302 (2017).
Reyneke, B., Dobrowsky, P. H., Ndlovu, T., Khan, S. & Khan, W. EMA-qPCR to monitor the efficiency of a closed-coupled solar pasteurization system in reducing Legionella contamination of roof-harvested rainwater. Sci. Total Environ. 553, 662–670 (2016).
Islam, M. F. & Johnston, R. B. Household pasteurization of drinking-water: the Chulli water-treatment system. J. Health Popul. Nutr. 24, 356–362 (2006).
Macomber P. S. H. Guidelines on Rainwater Catchment Systems for Hawaii. (College of Tropical Agriculture and Human Resources, and U.S. Department of Agriculture, Mänoa, Honolulu, Hawaii, 2004).
Lye, D. J. Bacterial levels in cistern water systems of northern Kentucky. J. Am. Water Resour. Assoc. 23(6), 1063–1068 (1987).
Stump, B., McBroom, M. & Darville, R. Demographics, practices and water quality from domestic potable rainwater harvesting systems. J. Water Suppl. Res. Technol. 61, 261–271 (2012).
Thomas, R. B., Kirisits, M. J., Lye, D. J. & Kinney, K. A. Rainwater harvesting in the United States: a survey of common system practices. J. Clean. Prod. 75, 166–173 (2014).
Lee, J. Y., Yang, J.-S., Han, M. & Choi, J. Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water sources. Sci. Total Environ. 408, 896–905 (2010).
Pinfold, J. V., Horan, N. J., Wirojanagud, W. & Mara, D. The bacteriological quality of rainjar water in rural Northeast Thailand. Water Res. 27, 297–302 (1993).
Horak, H. M. et al. Microbial and metal water quality in rain catchments compared with traditional drinking water sources in the East Sepik Province, Papua New Guinea. J. Water Health 8, 126–138 (2010).
Kirs, M. et al. Rainwater harvesting in American Samoa: current practices and indicative health risks. Environ. Sci. Pollut. Res. 24, 12384–12392 (2017).
Savill, M. G. et al. Enumeration of Campylobacter in New Zealand recreational and drinking waters. J. Appl. Microbiol. 91, 38–46 (2001).
Uba, B. N. & Aghogho, O. Rainwater quality from different roof catchments in the Port Harcourt District, Rivers State, Nigeria. J. Water Supply Res. T-Aqua. 49, 281–288 (2000).
Dobrowsky, P. H., Kwaadsteniet, M. D., Cloete, T. E. & Khan, W. Distribution of indigenous bacterial pathogens and potential pathogens associated with roof-harvested rainwater. Appl. Environ. Microbiol. 80, 2307–2316 (2014).
Broadhead, A. N., Negron-Alvira, A., Baez, L. A., Hazen, T. C. & Canoy, M. Occurrence of Legionella Species in tropical rain water cisterns. Carrib. J. Sci. 24, 71–73 (1988).
Xavier, R. P. et al. Microbiological quality of drinking rainwater in the inland region of Pajeu, Pernambuco, Northeast Brazil. Rev. Inst. Med. Trop. Sao Paulo 53, 121–124 (2011).
Abo-Shehada, M. N., Hibdyia, M. & Saiah, A. Prevalence of Cryptosporidium parvum in private drinking water cisterns in Bani-Kenanah distric, Northern Jordan. Int. J. Environ. Health Res. 14, 351–358 (2017).
Waso, M. et al. Abundance of Naegleria fowleri in roof-harvested rainwater tank samples from two continents. Environ. Sci. Pollut. Res. 25, 5700–5710 (2017).
Gardner, T., Baisden, J. & Millar, G. in Sustainable Water in the Urban Environment, 2004. Conference, Brisbane (2004).
Keithley, S. E., Kiristis, M. J. & Kinney, K. A. The effect of treatment on the quality of harvested rainwater. M.Sc. Thesis. University of Texas at Austin (2012).
Lantagne, D. S., Blount, B. C., Cardinali, F. & Quick, R. Disinfection by-product formation and mitigation strategies in point-of-use chlorination of turbid and non-turbid waters in western Kenya. J. Water Health 6, 67–82 (2008).
Moreira Neto, R. F., Calijuri, M. L., de Castro Carvalho, I. & da Fonseca Santiago, A. Rainwater treatment in airports using slow sand filtration followed by chlorination: efficiency and costs. Resour. Conserv. Recycl. 65, 124–129 (2012).
Islam, M. M., Chou, F. N. F., Kabir, M. R. & Liaw, C. H. Rainwater: a potential alternative source for scarce safe drinking and arsenic contaminated water in Bangladesh. Water Resour. Manage. 24, 3987–4008 (2010).
Dobrowsky, P. H. et al. Efficiency of microfiltration systems for the removal of bacterial and viral contaminants from surface and rainwater. Water Air Soil Pollut. 226, 1–14 (2015b).
Areerachakul, N. et al. Submerged membrane system with biofilter as a treatment to rainwater. Water Air Soil Pollut. 9, 431–438 (2009).
Ding, A. et al. A low pressure gravity-driven membrane filtration (GDM) system for rainwater recycling: Flux stabilization and removal performance. Chemosphere 172, 21–28 (2017).
Naddeo, V., Scannapieco, D. & Belgiorno, V. Enhanced drinking water supply through harvested rainwater treatment. J. Hydrol. 498, 287–291 (2013).
Nolde, E. Possibilities of rainwater utilization in densely populated areas including precipitation runoffs from traffic surfaces. Desalination 215, 1–11 (2007).
Amin, M. T. & Han, M. Y. Roof-harvested rainwater for potable purposes: application of solar disinfection (SODIS) and limitations. Water Sci. Technol. 60, 419–431 (2009).
Author information
Authors and Affiliations
Contributions
W.A. and K.H. designed the structure of the manuscript. K.H., B.R., M.W., T.C., T.N., W.K., K.D., E.R., F.M., drafted the first version of the manuscript. C.H. has made suggestions and revisions to enhance the clarity of the overall contents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamilton, K., Reyneke, B., Waso, M. et al. A global review of the microbiological quality and potential health risks associated with roof-harvested rainwater tanks. npj Clean Water 2, 7 (2019). https://doi.org/10.1038/s41545-019-0030-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-019-0030-5
This article is cited by
-
Water quality and microecosystem of water tanks in karst mountainous area, Southwest China
Environmental Science and Pollution Research (2024)
-
Decision making for implementing non-traditional water sources: a review of challenges and potential solutions
npj Clean Water (2023)
-
Microbiological characterization of stormwater in a high-income neighborhood in Rio de Janeiro, Brazil
Environmental Monitoring and Assessment (2022)
-
Quality assessment of harvested rainwater and seasonal variations in the southwest coastal area, Bangladesh
Environmental Earth Sciences (2021)
-
Physicochemical, microbiological quality, and risk assessment of water consumed by a quilombola community in midwestern Brazil
Environmental Science and Pollution Research (2021)