Abstract
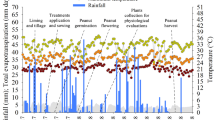
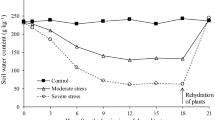
The objective of the work is to evaluate whether Bradyrhizobium sp. SEMIA6144 and Azospirillum brasilense Az39 can be used for inoculation to mitigate the negative effect of water restriction on growth of Arachis hypogaea. In this study, nitrogenase activity was determined by measuring the H2 evolution in an open-flow system, and N and C concentration in plants were determined by Elemental Analyzer. Lipid peroxidation and polyamines (PA) levels were analyzed by HPLC. The results showed that the restrictive water condition (RWC) caused an 80% inhibition of the N fixation rate. Although both single and double inoculation favored peanut growth under RWC, the inoculation with SEMIA6144 was better. Peanut plants have higher numbers of nodules in the roots when inoculated with SEMIA6144 in the absence of Az39, although it was observed that the inoculation with Az39 favored root development thus allowing the appearance of more infection sites in peanut roots. In double inoculation, the demand for N in the peanut was met with greater effectiveness. PAs found in leaves were putrescine, spermidine, and spermine. The results show that SEMIA6144 inoculation reversed the negative effects of RWC on growth and nodulation peanut parameters. Simultaneous application of SEMIA6144 and Az39 improved early nodulation, efficiency in N fixation and total N, thus increasing the tolerance of A. hypogaea to RWC. Our findings provide new insights in the context of mixed inoculation and improvement of peanut production in a limited water environment.





Similar content being viewed by others
References
Albareda M, Dardanelli M, Sousa C, Megías M, Temprano F, Rodriguez-Navarro D (2006) Factors affecting the attachment of rhizospheric bacteria to bean and soybean roots. FEMS Microbiol Lett 259:68–72
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C (2010) Polyamines: molecules with regulatory functions in plant biotic stress tolerance. Planta 231:1237–1249
Askary M, Mostajeran A, Amooaghaei R, Mostajeran M (2009) Influence of the co-inoculation Azospirillum brasilense and Rhizobium meliloti. plus 2,4-D on grain yield and N, P, K content of Triticum aestivum (Cv. Baccros and Mahdavi). Am Eurasian J Agric Environ Sci 5:296–307
Bacon C, Hinton D (2006) Bacterial endophytes: the endophytic niche, its occupants, and its utility. In: Gnanamanickam S (ed) Plant-associated bacteria. Springer, Dordrecht, pp 155–194
Barberis N, Bongiovanni R (2015) Resultado económico esperado de la agricultura, campaña agrícola. INTA, Informe económico para el productor I: Departamento Río Segundo, Córdoba, INTA. https://inta.gob.ar/sites/default/files/script-tmp-inta_informe_econmico_para_el_productor_i___mayo_2015.pdf
Barbosa D, Brito S, Fernandes P, Fernandes–Junior P, Lima L (2018) Can Bradyrhizobium strains inoculation reduce water deficit effects on peanuts? World J Microbiol Biotechnol 34:87. https://doi.org/10.1007/s11274-018-2474-z
Barea J, Pozo M, Azcón R, Azcón-Aguilar (2005) Microbial cooperation in the rhizosphere. J Exp Bot 56:1761–1778
Bashan L (2011) Cell–cell interaction in the eukaryote-prokaryote model of the microalgae Chlorella vulgaria and the bacterium Azospirillum brasilense immobilized in polymer bead. J Phycol 47:1350–1359
Berggren I, Alström JW, van Vuurde A, Martensson AN (2005) Rhizoplane colonization of peas by Rhizobium leguminosarum bv. viceae and a deleterious Pseudomonas putida. FEMS Microbiol Ecol 52:71–78
Bianucci E, Furlan A, Rivadeneira J, Sobrino-Plata J, Carpena-Ruiz R, Tordable C, Fabra A, Hernández B, Castro S (2013) Influence of cadmium on the symbiotic interaction established between peanut (Arachis hypogaea L.) and sensitive or tolerant bradyrhizobial strains. J Environ Manag 130:126–134
Boogerd F, van Rossum D (1997) Nodulation of groundnut by Bradyrhizobium: a simple infection process by crack entry. FEMS Microbiol Rev 21:5–27
Brusamarello-Santos LC, Gilard FE, Brule L, Quillere I, Gourion B, Ratet P, Maltempi de Souza E, Lea P, Hirel B (2016) Metabolic profiling of two maize (Zea mays L.) inbred lines inoculated with the nitrogen fixing plant-interacting bacteria Herbaspirillum seropedicae and Azospirillum brasilense. PLOS ONE 12:1–19
Cassan F, Diaz-Zorita M (2016) Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biol Biochem 103:117–130
Celikkol A, Ercan M, Kavas L, Yildiz C, Yilmaz H, Oktem A, Yucel M (2010) Drought induced oxidative damage and antioxidant responses in peanut (Arachis hypogaea L.) seedlings. Plant Growth Regul 61:21–22
Cesari A, Paulucci N, Biasutti M, Reguera Y, Gallarato L, Kilmurray C, Dardanelli M (2016) Reorganization of Azospirillum brasilense cell membrane is mediated by lipid composition adjustment to maintain optimal fluidity during water deficit. J Appl Microbiol 120:185–194
Cesari A, Paulucci N, Biasutti M, Morales G, Dardanelli M (2018) Changes in the lipid composition of Bradyrhizobium cell envelope reveal a rapid response to water deficit involving lysophosphatidylethanolamine synthesis from phosphatidylethanolamine in outer membrane. Res Microbiol 169:303–312
Chibeba A, Guimarães M, Rodrigues Brito O, Nogueira M, Araujo R (2015) Co-inoculation of soybean with Bradyrhizobium and Azospirillum promotes early nodulation. Am J Plant Sci 6:1641–1649
Cytryn E, Sangurdekar D, Streeter J, Franck W, Chang W, Stacey G, Emerich D, Joshi T, Xu D, Sadowsky M (2007) Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation-induced stress. J Bacteriol 189:6751–6762
Dardanelli M, Fernández F, Espuny M, Rodríguez Carvajal M, Soria Díaz M, Gil Serrano M, Okon Y, Megías M (2008) Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol Biochem 40:2713–2721
Davey A, Simpson R (1990) Nitrogen fixation by subterra-nean clover at varying stages of nodule dehydration. J Exp Bot 41:1175–1187
Di Rienzo J, Casanoves F, Balzarini M, Gonzalez L, Tablada M, Robledo C (2016) InfoStat version. InfoStat Group. National University of Córdoba, Argentina. http://www.infostat.com.ar
Döbereiner J, Day J (1976) Associative symbioses in tropical grasses: characterization of microorganisms and dinitrogen-fixing sites. In: Nyman W (ed) Proceedings of the 1st international symposium on nitrogen fixation, vol 2. Pullman Washington State University Press, Washington, pp 518–538
Estevez J, Dardanelli M, Megias M, Rodríguez-Navarro D (2009) Symbiotic performance of common bean and soybean coinoculated whith rhizobia and Chryseobacterium Aur9 under moderate saline conditions. Symbiosis 49:29–36
Fabra A, Castro S, Taurian T, Angelini J, Ibañez F, Dardanelli M, Tonelli M, Bianucci E, Valetti L (2010) Interaction among Arachis hypogaea L. (peanut) and beneficial soil microorganisms: how much is it known? Crit Rev Microbiol 36:179–194
Fernandez E, Giayetto O (2017) El cultivo del maní en Córdoba 2da Edición. Universidad Nacional de Río Cuarto. Libro digital. https://www.produccionvegetalunrc.org/docs/ECMC_2.pdf
Flores HE, Galston AW (1982) Analysis of polyamines in higher-plants by high performance liquid-chromatography. Plant Physiol 69:701–706
Fujihara S (2009) Biogenic amines in rhizobia and legume root nodules. Microbes Environ 24:1–13
Fujihara S, Yoneyama T (1993) Effect of pH and osmotic stress on cellular polyamine contents in the soybean rhizobia Rhizobium fredii P220 and Bradyrhizobium japonicum A1017. Appl Environ Microbiol 59:1104–1109
Gamarnik A, Frydman R (1991) Cadaverine, an essential diamine for the normal root development of germinating soybean (Glycine max) seeds. Plant Physiol 97:778–785
Gill S, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Grant O (2012) Understanding and exploiting the impact of drought stress on plant physiology. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer Science Business Media, España, pp 89–104
Green L, Emerich D (1999) Light microscopy of early stages in the symbiosis of soybean with a delayed-nodulation mutant of Bradyrhizobium japonicum. J Exp Bot 50:1577–1585
Groppa M (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Hodges D, DeLong J, Forney C, Prange R (1999) Improving the thiobarbituric acid-reactive-substances assay for stimulating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Floor 207:604–611
INTA (2017) Producción de Maní en la zona centro-norte de Córdoba. https://inta.gob.ar/documentos/produccion-de-mani-en-la-zona-centro-norte-de-cordoba-evaluacion-de-la-respuesta-a-la-aplicacion-de-tratamiento-combinado-de-fungicida-mas-inoculante-en-semillas-versus-tratamiento-en-surco
INTA (2018) Informe de sequía en el territorio sudoeste de Córdoba. Campaña 2017–2018. https://inta.gob.ar/sites/default/files/inta_informe_sequia_pretso_cba.pdf
Jesus E, Leite R, Amaral Bastos R, da Silva Aragão O, Araújo A (2017) Co-inoculation of Bradyrhizobium stimulates the symbiosis efficiency of Rhizobium with common bean. Plant Soil 425:201–215
Jiménez-Bremont J, Marina M, Guerrero-González M, Rossi F, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz O, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95:1–14
King C, Purcell L (2005) Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol 137:1389–1396
Kris-Etherton P, Hu F, Ros E (2008) The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr 138:1746–1751
Liu B, Sutton A, Sternglanz R (2005) A yeast polyamine acetyltransferase. J Biol Chem 280:16659–16664
Liu J, Kitashiba H, Wang J, Ban Y, Moriguchi T (2007) Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol 24:117–126
López-Gómez M, Cobos-Porras L, Hidalgo-Castellanos J, Lluch C (2014) Occurrence of polyamines in root nodules of Phaseolus vulgaris in symbiosis with Rhizobium tropici in response to salt stress. Phytochemistry 107:32–41
Lucas Garcia J, Barbas C, Provanza A, Barrientos M, Gutierrez Mañero F (2001) Low molecular weight organic acids and fatty acids in root exudates of two Lupinus cultivars at flowering and fruiting stages. Phytochem Anal 12:305–311
Medeot D, Sohlenkamp C, Dardanelli M, Geiger O, García de Lema M, López-Lara IM (2009) Phosphatidylcholine levels of peanut-nodulating Bradyrhizobium sp. SEMIA6144 affect cell size and motility. FEMS Microbiol Lett 303:123–131
Mhamdi R, Nouairi I, Hammouda T, Mhamdi R, Mhadhbi H (2014) Growth capacity and biochemical mechanisms involved in rhizobia tolerance to salinity and water deficit. J Basic Microbiol 54:1–11
Miles A, Misra S, Irwin J (1938) The estimation of the bactericidal power of the blood. J Hyg 38:732–749
Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M (2015) Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol 427:3389–3406
Niemi K, Haggman H, Sarjala T (2001) Effects of exogenous diamines on the interaction between ectomycorrhizal fungi and adventitious root formation in Scots pine in vitro. Tree Physiol 22:373–381
Okon Y, Itzigsohn R (1995) The development of Azospirrillum as a commercial inoculant for improving crop yields. Biotechnol Adv 13:415–424
Paulucci N, Gallarato L, Reguera Y, Vicario J, Cesari A, García de Lema M, Dardanelli M (2015) Arachis hypogaea PGPR isolated from Argentine soil modifies its lipids components in response to temperature and salinity. Microbiol Res 173:1–9
Pereyra G, Hartmann H, Michalzik B, Ziegler W, Trumbore S (2015) Influence of rhizobia inoculation on biomass gain and tissue nitrogen content of Leucaena leucocephala seedlings under drought. Forests 6:3686–3703
Purcell L, King C (1996) Drought and nitrogen source effects on nitrogen nutrition, seed growth, and yield in soybean. J Plant Nutr 19:969–993
Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G (2009) Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. Ann Bot 104:1255–1444
Rider J, Hacker C, Mackintosh A, Pegg P, Woster R (2007) Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 33:231–240
Rodriguez Cáceres E (1982) Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44:990–991
Romero-Puertas MC, Corpas FJ, Rodriguez-Serrano M, Gomez M, del Río LA, Sandalio LM (2004) Differential expression and regulation of antioxidative enzymes by Cd in pea plants. J Plant Physiol 164:1346–1357
Schubert S, Serraj R, Plies-Balzer E, Mengel K (1995) Effect of drought stress on growth, sugar concentrations and aminoacid accumulation in N2-fixing alfalfa (Medicago sativa). J Plant Physiol 146:541–546
Serraj R, Valdez V, Denison F, Sinclair T (1999) Symbiosis N2 fixation response to drought. J Exp Bot 50:143–157
Sharma IP, Chandra S, Kumar N, Chandra D (2017) PGPR: heart of soil and their role in soil fertility. In: Meena V, Mishra P, Bish J, Pattanayak A (eds) Agriculturally important microbes for sustainable agriculture, Vol I. Springer, Singapore, pp 51–67
Streeter J (2003) Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environ 26:1199–1220
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley–powdery mildew interaction. Plant J 11:1187–1194
van Brussel A, Planque K, Quispel A (1977) The wall of Rhizobium leguminosarum in bacteroid and free-living forms. J Gen Microbiol 101:51–56
Vicario J, Primo E, Dardanelli M, Giordano W (2015) Promotion of peanut growth by coinoculation with selected strains of Bradyrhizobium and Azospirillum. J Plant Growth Reg 35:413–419
Vincent J (1970) A manual for the practical study of root nodule bacteria. Internat. Biol. Progr. Handbook Nº 15. Blackwell Scientific Publications Ltd, Oxford
Witty J, Minchin F (1998) Method for the continuous measurement of O2 consumption and H2 production by nodulated legume root system. J Exp Bot 49:1041–1047
Zahran H (1998) Structure of root nodules and nitrogen fixation in Egyptian wild herb legumes. Biol Plant 41:575–585
Zapata P, Serrano M, Pretel M, Amoros A, Botella M (2004) Polyamines and ethylene changes during germination of different plant species under salinity. Plant Sci 167:781–788
Zheng Z (2009) Carbon and nitrogen nutrient balance signaling in plants. Plant Signal Behav 4:584–591
Acknowledgements
Financial assistance was provided by PIP CONICET 112-201101-00309, PIP CONICET 112-201501-00232, and SECYT UNRC Nº 161/16. A.B.C. is a fellow of CONICET-Argentina. M.S.D. and N.S.P are members of the Research Career of CONICET-Argentina. This work also has been supported by the Andalusian Research Program (AGR-139). Asociación Universitaria Iberoamericana de Postgrado (AUIP). International mobility scholarship between Andalusian and Iberoamerican universities. We thank Dr. Elena Fernandez for advice on statistical analysis of the data. Finally, we are also grateful to editors and anonymous reviewers for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cesari, A.B., Paulucci, N.S., López-Gómez, M. et al. Performance of Bradyrhizobium and Bradyrhizobium–Azospirillum in Alleviating the Effects of Water-Restrictive Conditions During the Early Stages of Arachis hypogaea Growth. J Plant Growth Regul 38, 1362–1374 (2019). https://doi.org/10.1007/s00344-019-09939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09939-4