Abstract
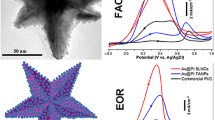
To address the insufficient electrocatalytic activity and stability of formic acid oxidation reaction (FAOR) electrocatalysts, as well as their high cost, we herein demonstrate the facile hydrothermal synthesis of ultrathin AgPt alloy nanowires using amine-terminated poly(N-isopropylacrylamide) (PNIPAM-NH2) as a structure-directing agent. The initial generation of AgCl precipitates, subsequent formation of AgPt nanoparticles, and their oriented attachment account for the formation of ultrathin AgPt alloy nanowires. Benefiting from their unique 1D anisotropy and alloyed composition, the prepared ultrathin AgPt nanowires exhibit a superior electrocatalytic activity and better CO tolerance for the FAOR, reaching a 1.6-fold and 3.7-fold higher specific current density than AgPt nanoparticles and a commercial Pt black catalyst, respectively. Additionally, the ultrathin AgPt alloy nanowires manifest a superior electrochemical stability and structural robustness during electrocatalysis, making them a promising FAOR electrocatalyst. This work not only provides a reliable strategy for the synthesis of noble metal-based ultrathin nanowires, but also opens an avenue towards the rational design of efficient electrocatalysts for fuel cell systems.

Similar content being viewed by others
References
Li, C. L.; Jiang, B.; Miyamoto, N.; Kim, J. H.; Malgras, V.; Yamauchi, Y. Surfactant-directed synthesis of mesoporous Pd films with perpendicular mesochannels as efficient electrocatalysts. J. Am. Chem. Soc. 2015, 137, 11558–11561.
Feng, L. G.; Chang, J. F.; Jiang, K.; Xue, H. G.; Liu, C. P.; Cai, W. B.; Xing, W.; Zhang, J. J. Nanostructured palladium catalyst poisoning depressed by cobalt phosphide in the electro-oxidation of formic acid for fuel cells. Nano Energy 2016, 30, 355–361.
Gralec, B.; Lewera, A. Catalytic activity of unsupported Pd-Pt nanoalloys with low Pt content towards formic acid oxidation. Appl. Catal. B 2016, 192, 304–310.
Wang, R. Y.; Liu, J. G.; Liu, P.; Bi, X. X.; Yan, X. L.; Wang, W. X.; Meng, Y. F.; Ge, X. B.; Chen, M. W.; Ding, Y. Ultra-thin layer structured anodes for highly durable low-Pt direct formic acid fuel cells. Nano Res. 2014, 7, 1569–1580.
Kang, Y. J.; Murray, C. B. Synthesis and electrocatalytic properties of cubic Mn-Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 2010, 132, 7568–7569.
Yang, S.; Lee, H. Atomically dispersed platinum on gold nano-octahedra with high catalytic activity on formic acid oxidation. ACS Catal. 2013, 3, 437–443.
Gong, M. X.; Li, F. M.; Yao, Z. G.; Zhang, S. Q.; Dong, J. W.; Chen, Y.; Tang, Y. W. Highly active and durable platinum-lead bimetallic alloy nanoflowers for formic acid electrooxidation. Nanoscale 2015, 7, 4894–4899.
Choi, S. I.; Herron, J. A.; Scaranto, J.; Huang, H. W.; Wang, Y.; Xia, X. H.; Lv, T.; Park, J.; Peng, H. C.; Mavrikakis, M. et al. A comprehensive study of formic acid oxidation on palladium nanocrystals with different types of facets and twin defects. ChemCatChem 2015, 7, 2077–2084.
Saleem, F.; Xu, B.; Ni, B.; Liu, H. L.; Nosheen, F.; Li, H. Y.; Wang, X. Atomically thick Pt-Cu nanosheets: Self-assembled sandwich and nanoring-like structures. Adv. Mater. 2015, 27, 2013–2018.
Jiang, K.; Xu, K.; Zou, S. Z.; Cai, W. B. B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid-formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864.
Liu, D.; Xie, M. L.; Wang, C. M.; Liao, L. W.; Qiu, L.; Ma, J.; Huang, H.; Long, R.; Jiang, J.; Xiong, Y. J. Pd-Ag alloy hollow nanostructures with interatomic charge polarization for enhanced electrocatalytic formic acid oxidation. Nano Res. 2016, 9, 1590–1599.
Xu, L.; Luo, Z. M.; Fan, Z. X.; Zhang, X.; Tan, C. L.; Li, H.; Zhang, H.; Xue, C. Triangular Ag-Pd alloy nanoprisms: Rational synthesis with high-efficiency for electrocatalytic oxygen reduction. Nanoscale 2014, 6, 11738–11743.
Kuang, Y.; Cai, Z.; Zhang, Y.; He, D. S.; Yan, X. L.; Bi, Y. M.; Li, Y. P.; Li, Z. Y.; Sun, X. M. Ultrathin dendritic Pt3Cu triangular pyramid caps with enhanced electrocatalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 17748–17752.
Ge, J. J.; He, D. S.; Bai, L.; You, R.; Lu, H. Y.; Lin, Y.; Tan, C. L.; Kang, Y. B.; Xiao, B.; Wu, Y. et al. Ordered porous Pd octahedra covered with monolayer Ru atoms. J. Am. Chem. Soc. 2015, 137, 14566–14569.
Chen, S.; Su, H. Y.; Wang, Y. C.; Wu, W. L.; Zeng, J. Sizecontrolled synthesis of platinum-copper hierarchical trigonal bipyramid nanoframes. Angew. Chem., Int. Ed. 2015, 54, 108–113.
Jia, Y. Y.; Jiang, Y. Q.; Zhang, J. W.; Zhang, L.; Chen, Q. L.; Xie, Z. X.; Zheng, L. S. Unique excavated rhombic dodecahedral PtCu3 alloy nanocrystals constructed with ultrathin nanosheets of high-energy {110} facets. J. Am. Chem. Soc. 2014, 136, 3748–3751.
Park, J.; Liu, J. Y.; Peng, H. C.; Figueroa-Cosme, L.; Miao, S.; Choi, S. I.; Bao, S. X.; Yang, X.; Xia, Y. N. Coating Pt–Ni octahedra with ultrathin Pt shells to enhance the durability without compromising the activity toward oxygen reduction. ChemSusChem 2016, 9, 2209–2215.
Ye, S. H.; Feng, J. X.; Wang, A. L.; Xu, H.; Li, G. R. Multi-layered Pt/Ni nanotube arrays with enhanced catalytic performance for methanol electrooxidation. J. Mater. Chem. A 2015, 3, 23201–23206.
Qiu, P. T.; Lian, S. M.; Yang, G.; Yang, S. C. Halide ioninduced formation of single crystalline mesoporous PtPd bimetallic nanoparticles with hollow interiors for electrochemical methanol and ethanol oxidation reaction. Nano Res. 2017, 10, 1064–1077.
Peng, Z. M.; You, H. J.; Yang, H. An Electrochemical approach to PtAg alloy nanostructures rich in Pt at the surface. Adv. Funct. Mater. 2010, 20, 3734–3741.
Jiang, X.; Yan, X. X.; Ren, W. Y.; Jia, Y. F.; Chen, J. N.; Sun, D. M.; Xu, L.; Tang, Y. W. Porous AgPt@Pt nanooctahedra as an efficient catalyst toward formic acid oxidation with predominant dehydrogenation pathway. ACS Appl. Mater. Interfaces 2016, 8, 31076–31082.
Fu, G. T.; Ma, R. G.; Gao, X. Q.; Chen, Y.; Tang, Y. W.; Lu, T. H.; Lee, J. M. Hydrothermal synthesis of Pt–Ag alloy nano-octahedra and their enhanced electrocatalytic activity for the methanol oxidation reaction. Nanoscale 2014, 6, 12310–12314.
Mahmood, A.; Saleem, F.; Lin, H. F.; Ni, B.; Wang, X. Crystallinity-induced shape evolution of Pt-Ag nanosheets from branched nanocrystals. Chem. Commun. 2016, 52, 10547–10550.
Cao, X.; Han, Y.; Gao, C. Z.; Huang, X. M.; Xu, Y.; Wang, N. PtAg nanowires: Facile synthesis and their applications as excellent oxygen reduction electrocatalysts for label-free electrochemical immunoassay. J. Mater. Chem. A 2013, 1, 14904–14909.
Cao, X.; Wang, N.; Han, Y.; Gao, C. Z.; Xu, Y.; Li, M. X.; Shao, Y. H. PtAg bimetallic nanowires: Facile synthesis and their use as excellent electrocatalysts toward low-cost fuel cells. Nano Energy 2015, 12, 105–114.
Koenigsmann, C.; Sutter, E.; Chiesa, T. A.; Adzic, R. R.; Wong, S. S. Highly enhanced electrocatalytic oxygen reduction performance observed in bimetallic palladium-based nanowires prepared under ambient, surfactantless conditions. Nano Lett. 2012, 12, 2013–2020.
Xu, L.; Yang, Y.; Hu, Z. W.; Yu, S. H. Comparison study on the stability of copper nanowires and their oxidation kinetics in gas and liquid. ACS Nano 2016, 10, 3823–3834.
Huang, X. Q.; Zhao, Z. P.; Chen, Y.; Chiu, C. Y.; Ruan, L. Y.; Liu, Y.; Li, M. F.; Duan, X. F.; Huang, Y. High density catalytic hot spots in ultrafine wavy nanowires. Nano Lett. 2014, 14, 3887–3894.
Fan, Z. X.; Luo, Z. M.; Huang, X.; Li, B.; Chen, Y.; Wang, J.; Hu, Y. L.; Zhang, H. Synthesis of 4H/fcc noble multimetallic nanoribbons for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2016, 138, 1414–1419.
Liu, Z. Q.; Cheng, H.; Li, N.; Ma, T. Y.; Su, Y. Z. ZnCo2O4 quantum dots anchored on nitrogen-doped carbon nanotubes as reversible oxygen reduction/evolution electrocatalysts. Adv. Mater. 2016, 28, 3777–3784.
Zhao, W. Y.; Huang, D. B.; Yuan, Q.; Wang, X. Sub-2.0-nm Ru and composition-tunable RuPt nanowire networks. Nano Res. 2016, 9, 3066–3074.
Hong, W.; Wang, J.; Wang, E. Dendritic Au/Pt and Au/PtCu nanowires with enhanced electrocatalytic activity for methanol electrooxidation. Small 2014, 10, 3262–3265.
Feng, Y. G.; Bu, L. Z.; Guo, S. J.; Guo, J.; Huang, X. Q. 3D platinum–lead nanowire networks as highly efficient ethylene glycol oxidation electrocatalysts. Small 2016, 12, 4464–4470.
Xia, B. Y.; Wu, H. B.; Yan, Y.; Lou, X. W.; Wang, X. Ultrathin and ultralong single-crystal platinum nanowire assemblies with highly stable electrocatalytic activity. J. Am. Chem. Soc. 2013, 135, 9480–9485.
Ding, J. B.; Bu, L. Z.; Zhang, N.; Yao, J. L.; Huang, Y.; Huang, X. Q. Facile synthesis of ultrathin bimetallic PtSn wavy nanowires by nanoparticle attachment as enhanced hydrogenation catalysts. Chemistry 2015, 21, 3901–3905.
Wu, L. P.; Liu, Z. Y.; Xu, M.; Zhang, J.; Yang, X. Y.; Huang, Y. D.; Lin, J.; Sun, D. M.; Xu, L.; Tang, Y. W. Facile synthesis of ultrathin Pd-Pt alloy nanowires as highly active and durable catalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 6805–6813.
Dai, L.; Mo, S. G.; Qin, Q.; Zhao, X. J.; Zheng, N. F. Carbon monoxide-assisted synthesis of ultrathin PtCu3 alloy wavy nanowires and their enhanced electrocatalysis. Small 2016, 12, 1572–1577.
Li, M. F.; Zhao, Z. P.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q. H.; Gu, L.; Merinov, B. V.; Lin, Z. Y. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419.
Nam, I.; Kim, N. D.; Kim, G. P.; Park, J.; Yi, J. One step preparation of Mn3O4/graphene composites for use as an anode in Li ion batteries. J. Power Sources 2013, 244, 56–62.
Liu, H.; Ye, F.; Yao, Q. F.; Cao, H. B.; Xie, J. P.; Lee, J. Y.; Yang, J. Stellated Ag-Pt bimetallic nanoparticles: An effective platform for catalytic activity tuning. Sci. Rep. 2014, 4, 3969.
Yang, J.; Ying, J. Y. Nanocomposites of Ag2S and noble metals. Angew. Chem., Int. Ed. 2011, 50, 4637–4643.
Ruan, L. Y.; Zhu, E. B.; Chen, Y.; Lin, Z. Y.; Huang, X. Q.; Duan, X. F.; Huang, Y. Biomimetic synthesis of an ultrathin platinum nanowire network with a high twin density for enhanced electrocatalytic activity and durability. Angew. Chem., Int. Ed. 2013, 52, 12577–12581.
Peng, Z. M.; You, H. J.; Yang, H. Composition-dependent formation of platinum silver nanowires. ACS Nano 2010, 4, 1501–1510.
Zhang, W. Q.; Yang, J. Z.; Lu, X. M. Tailoring galvanic replacement reaction for the preparation of Pt/Ag bimetallic hollow nanostructures with controlled number of voids. ACS Nano 2012, 6, 7397–7405.
Herrero, E.; Buller, L. J.; Abruña, H. D. Underpotential deposition at single crystal surfaces of Au, Pt, Ag and other materials. Chem. Rev. 2001, 101, 1897–1930.
Teng, X. W.; Feygenson, M.; Wang, Q.; He, J. Q.; Du, W. X.; Frenkel, A. I.; Han, W. Q.; Aronson, M. Electronic and magnetic properties of ultrathin Au/Pt nanowires. Nano Lett. 2009, 9, 3177–3184.
Wang, C.; Hu, Y. J.; Lieber, C. M.; Sun, S. H. Ultrathin Au nanowires and their transport properties. J. Am. Chem. Soc. 2008, 130, 8902–8903.
Yu, X. F.; Wang, D. S.; Peng, Q.; Li, Y. D. Pt-M (M=Cu, Co, Ni, Fe) nanocrystals: From small nanoparticles to wormlike nanowires by oriented attachment. Chemistry 2013, 19, 233–239.
Ataee-Esfahani, H.; Skrabalak, S. E. Attachment-based growth: Building architecturally defined metal nanocolloids particle by particle. RSC Adv. 2015, 5, 47718–47727.
Liang, H. W.; Cao, X.; Zhou, F.; Cui, C. H.; Zhang, W. J.; Yu, S. H. A free-standing Pt-nanowire membrane as a highly stable electrocatalyst for the oxygen reduction reaction. Adv. Mater. 2011, 23, 1467–1471.
Sun, S. H.; Zhang, G. X.; Geng, D. S.; Chen, Y. G.; Banis, M. N.; Li, R. Y.; Cai, M.; Sun, X. L. Direct growth of single-crystal pt nanowires on Sn@CNT nanocable: 3D electrodes for highly active electrocatalysts. Chemistry 2010, 16, 829–835.
Fu, G. T.; Xia, B. Y.; Ma, R. G.; Chen, Y.; Tang, Y. W.; Lee, J. M. Trimetallic PtAgCu@PtCu core@shell concave nanooctahedrons with enhanced activity for formic acid oxidation reaction. Nano Energy 2015, 12, 824–832.
Qiu, X. Y.; Zhang, H. Y.; Wu, P. S.; Zhang, F. Q.; Wei, S. H.; Sun, D. M.; Xu, L.; Tang, Y. W. One-pot synthesis of freestanding porous palladium nanosheets as highly efficient electrocatalysts for formic acid oxidation. Adv. Funct. Mater. 2017, 27, 1603852.
Wang, Y.; Choi, S. I.; Zhao, X.; Xie, S. F.; Peng, H. C.; Chi, M. F.; Huang, C. Z.; Xia, Y. N. Polyol synthesis of ultrathin Pd nanowires via attachment-based growth and their enhanced activity towards formic acid oxidation. Adv. Funct. Mater. 2014, 24, 131–139.
Iyyamperumal, R.; Zhang, L.; Henkelman, G.; Crooks, R. M. Efficient electrocatalytic oxidation of formic acid using Au@Pt dendrimer-encapsulated nanoparticles. J. Am. Chem. Soc. 2013, 135, 5521–5524.
Nguyen, S. T.; Law, H. M.; Nguyen, H. T.; Kristian, N.; Wang, S. Y.; Chan, S. H.; Wang, X. Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media. Appl. Catal. B 2009, 91, 507–515.
Hammer, B.; Nørskov, J. K. Theoretical surface science and catalysis-calculations and concepts. Adv. Catal. 2000, 45, 71–129.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Nos. 21503111, 21576139 and 21376122) and Natural Science Foundation of Jiangsu Higher Education Institutions of China (No. 16KJB150020). The authors also thank National and Local Joint Engineering Research Center of Biomedical Functional Materials and a project sponsored by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, X., Fu, G., Wu, X. et al. Ultrathin AgPt alloy nanowires as a high-performance electrocatalyst for formic acid oxidation. Nano Res. 11, 499–510 (2018). https://doi.org/10.1007/s12274-017-1658-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1658-4