Abstract
Intensive fertilization of grasslands with cattle slurry can cause high environmental nitrogen (N) losses in form of ammonia (NH3), nitrous oxide (N2O), and nitrate (NO3−) leaching. Still, knowledge on short-term fertilizer N partitioning between plants and dinitrogen (N2) emissions is lacking. Therefore, we applied highly 15N-enriched cattle slurry (97 kg N ha−1) to pre-alpine grassland field mesocosms. We traced the slurry 15N in the plant-soil system and to denitrification losses (N2, N2O) over 29 days in high temporal resolution. Gaseous ammonia (NH3), N2 as well N2O losses at about 20 kg N ha−1 were observed only within the first 3 days after fertilization and were dominated by NH3. Nitrous oxide emissions (0.1 kg N ha−1) were negligible, while N2 emissions accounted for 3 kg of fertilizer N ha−1. The relatively low denitrification losses can be explained by the rapid plant uptake of fertilizer N, particularly from 0–4 cm depth, with plant N uptake exceeding denitrification N losses by an order of magnitude already after 3 days. After 17 days, total aboveground plant N uptake reached 100 kg N ha−1, with 33% of N derived from the applied N fertilizer. Half of the fertilizer N was found in above and belowground biomass, while at about 25% was recovered in the soil and 25% was lost, mainly in form of gaseous emissions, with minor N leaching. Overall, this study shows that plant N uptake plays a dominant role in controlling denitrification losses at high N application rates in pre-alpine grassland soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liquid cattle slurry has been increasingly replacing traditional fertilization with farmyard manure in mountain grasslands in Central Europe (Capriel 2013). It is well known that the use of cattle slurry can involve high gaseous and hydrological losses (Schröder et al. 2005), in particular in the form of ammonia (NH3) (Nyameasem et al. 2022) and nitrate (NO3−) (Chang and Entz 1996), and therefore, a relatively low plant N use efficiency and N sequestration in soils (Schlingmann et al. 2020; Schreiber et al. 2023; Zistl-Schlingmann et al. 2020). The overall high fertilizer N losses in grasslands therefore promote soil N mining, especially in organic matter-rich soils, where plant N nutrition is largely based on N from soil organic matter mineralization rather than from fertilizer (Schlingmann et al. 2020; Schreiber et al. 2023; Zistl-Schlingmann et al. 2020). The N losses impact air quality, human health, and biodiversity through NH3 emissions and N deposition, while NO3− leaching threatens groundwater quality (Fangueiro et al. 2014; Fu et al. 2017; Smith et al. 2002; Sutton et al. 2008). Slurry application also leads to nitrous oxide (N2O) emissions (Cardenas et al. 2019; Nyameasem et al. 2022; Rodhe et al. 2006), which are lower in calcareous pre-alpine soils due to pH-induced reduction of N2O to dinitrogen (N2) (Chen et al. 2015; Malique et al. 2021; Wu et al. 2020; Zistl-Schlingmann et al. 2019). In contrast to the potent greenhouse gas N2O, N2 emissions are environmentally benign (Butterbach-Bahl et al. 2013), but still denote fertilizer loss, impact grassland N balance and potentially accelerate soil N mining (Schlingmann et al. 2020; Wu et al. 2020; Zistl-Schlingmann et al. 2019, 2020). Furthermore, from a farmer's perspective, these emissions also translate into a financial loss, highlighting the economic impact alongside ecological concerns (Laborde et al. 2021). However, N2 emissions remain the greatest uncertainty in ecosystem nitrogen balances (Almaraz et al. 2020), particularly in grasslands (Zistl-Schlingmann et al. 2019). This is due to notorious challenges in measuring N2 emissions under real-world conditions (Friedl et al. 2020; Groffman 2012).
Buchen-Tschiskale et al. (2023) applied a 15N gas flux (15NGF) method under field conditions and reported N2 emissions to amount to up to 3 kg N ha−1 after addition of 67 kg N ha−1 of cattle slurry via different application techniques to winter wheat mesocosms from Southern Germany. Much higher N2 emissions of 11–19 kg N ha−1 were reported based on 15NGF measurements in grassland in Northern Ireland after the application of cattle slurry combined with large NO3− applications (together 65 kg N ha−1) (McGeough et al. 2012). In the latter studies, the addition of 15NO3− combined with the labile carbon (C) in cattle slurry might have stimulated N2 emissions. In contrast, in the study of Zistl-Schlingmann et al. (2019), all gaseous N losses were measured from intact grassland mesocosms after the addition of 51 kg N ha−1 of cattle slurry, with no further 15N addition needed, as N2 losses were directly measured in an artificial N2-free atmosphere established with the Helium substitution soil core technique. The latter study revealed total gaseous N losses equal to half of the slurry N addition. Thereby, N2 emissions with 31–42% of applied slurry-N were dominating N losses and even exceeded NH3 losses. In this study, however, plant N uptake of N was excluded due to dark chamber measurements, which might have led to an overestimation of denitrification due to impaired plant competition for N. This appears particularly important in temperate grasslands, which are rich in soil organic matter and show enormous productivity and plant N uptake, often even quantitatively exceeding N addition by fertilization (Fu et al. 2017). Despite such findings, which at first glance seem to indicate a high fertilizer N use efficiency by plants, several recent studies tracing 15N from labeled organic fertilizers into plant biomass in different pre-alpine grassland soils under different manure application techniques show the opposite. These studies found low fertilizer 15N recovery of only 3–38% in harvested plant biomass N 1–2 months after fertilization, indicating that N sources other than fertilizer contribute up to more than 90% of plant N uptake (Schlingmann et al. 2020; Schreiber et al. 2023; Zistl-Schlingmann et al. 2020). Also, various other 15N tracing studies in grasslands (Bardgett and Wardle 2003; Christian et al. 1997; Harrison et al. 2007, 2008; Hart et al. 1986; Rowlings et al. 2016; Scheiner et al. 2002; Xu et al. 2010) show high variance in total 15N recovery in the plant biomass, with recovery rates ranging from 2 to 50%.
Ammonium (NH4+) applied via organic fertilizer to grassland soils is subject to competition by plant uptake and microbial utilization such as nitrification, with the produced NO3− again either being taken up by plants or used by microbial processes such as denitrification (Butterbach-Bahl et al. 2013; Gao et al. 2023; Harrison et al. 2008). Hence, understanding the distribution of manure N between inorganic and organic forms, and how these forms are utilized by plants and microbes—referred to as manure N partitioning—is crucial to reducing N losses in the form of N2O and N2 and improving manure N use efficiency. In this context, a number of the aforementioned studies have investigated the fate of manure N using stable isotope techniques on time scales of months to years, and gaseous emissions of NH3 and N2O have also been increasingly well constrained. In contrast, N2 emissions from organic grassland fertilization have only been directly measured by a few studies, with very contrasting results (Friedl et al. 2017; McGeough et al. 2012; Zistl-Schlingmann et al. 2019). Furthermore, to our knowledge, no study has investigated the short-term partitioning of manure N in grasslands between plant N uptake and N2 emissions at shorter time scales relevant for a better understanding of the dynamics and magnitude of N2 emissions, i.e., a few days to weeks after fertilization. Given the potentially critical role of N2 in grassland N balances, these knowledge gaps severely hamper the development of management options aimed at reducing grassland N losses. Furthermore, knowledge of the quantitative role of plant N uptake versus N2 emissions will help to assess the extent to which dark chamber measurements of N2 emissions, as typically performed with the He soil core method, are biased by inactive grassland plants. Therefore, in this study, we applied highly 15N-enriched cattle slurry to pre-alpine grassland field mesocosms and followed the 15N signal with high temporal resolution in plant above and below ground biomass (AGB/BGB) and different vertical soil layers (temporal resolution: days 1, 3, 10, 17, 29 after fertilization). This was accompanied by direct measurements of gaseous N2 (using the 15NGF method) and N2O losses in daily temporal resolution, and the quantification of leached 15N after 29 days, allowing the calculation of a complete fertilizer 15N balance. Following this approach, the goals of the present study were to 1) assess the short-term dynamics of the partitioning of cattle slurry N in the plant-soil-system over 1 month in high temporal resolution; and 2) to quantify the role of N2 emissions versus plant N uptake in the fertilizer N balance after manure application under real field conditions. We hypothesized that a large short-term pulse of N2 emissions would substantially contribute to total gaseous N losses, potentially surpassing plant N uptake during the first days following fertilizer application. For longer time scales of weeks to one month after fertilization we expected plant N uptake of fertilizer-derived N to increase in importance compared to soil-derived N, thereby more and more exceeding fertilizer-derived N2 losses.
Material and methods
Study sites and experimental design
This study was conducted in the grasslands of the TERENO pre-alpine observatory in Southern Germany (Kiese et al. 2018). Sites are located in flat valley bottoms of the Northern Calcareous Alps. In total 30 intact plant-soil mesocosms of 16 cm diameter and 25 cm depth were excavated at three replicated plots (10 per plot) at the Graswang site (860 m a.s.l., (47°34′10.2’’N, 11°01′54.1’’E) in August 2016 and re-buried in situ within the Graswang site. The sampling site has a mean annual temperature (MAT) of 6.9 °C and mean annual precipitation (MAP) of 1347 mm (2014—2017) (Zistl-Schlingmann et al. 2020). The soil, characterized as a C- and N-rich Haplic Cambisol derived from limestone and dolomite, comprises 51% clay, 40% silt, and 9% sand (Kiese et al. 2018). Based on the mesocosm analyses of this study, bulk density was 0.60 ± 0.03 g cm−3 (0–4 cm); 0.64 ± 0.02 g cm−3 (4–10 cm) and 0.91 ± 0.02 g cm−3 (10–25 cm) (Garcia-Franco et al. 2024). The soil organic carbon (SOC) and total N (TN) content of the soil is 118 ± 10 and 12 ± 1 mg g−1 and (0–5 cm depth), and 98 ± 9 and 11 ± 1 mg g−1 (5–15 cm depth) (Garcia-Franco et al. 2024). Due to the carbonate buffer system, pH was measured at 7 in the 0–15 cm depth range (Unteregelsbacher et al. 2013). Despite large gross N mineralization and nitrification rates, both soil NH4+ and NO3− concentrations remain below 10 mg N kg−1 sdw throughout the growing season when there is no fertilization (Wang et al. 2016). The vegetation is dominated by the grasses Trisetetum flavescentis, Dactylis glomerata, Festuca pratensis und Festuca rubra and the herbaceous plants like Pimpinella major, Plantago lanceolata und Trifolium pratense (Schlingmann 2020). After four years of installation at the sampling site Graswang, for logistical reasons mesocosms were translocated on June 4, 2020, to a similar grassland site at Garmisch-Partenkirchen (730 m asl, close to the Alpine Campus of KIT, where MAT and MAP were 8.4 °C and 1360 mm, respectively (2014–2017, DWD). The experiment of this study started on June 22, 2020, with the application of 15N labeled slurry to all mesocosms. The experimental design is illustrated in Fig. 1. At days 3, 10, 17 and 29 after fertilization, entire mesocosms (N = 6 each) were destructively harvested to determine 15N excess recovery from the labeled slurry. Because of logistic and time constraints, the first harvest on day 1 after fertilization was a mesocosm subharvest of the day 3 cores. For this, we used a corer of 5 cm diameter (0–4 cm depth, and AGB) and 3 cm diameter (4–10 cm and 10–25 cm). Hence, each of the five harvests was followed by AGB ang BGB sampling, and soil sampling in 0–4, 4–10, and 10–25 cm depths as described below. Day 3 sampling was corrected for the missing soil mass and plant biomass due to the subsampling at day 1. Following this approach, data on soil and plant N pools and 15N recovery were obtained for days 1, 3, 10, 17 and 29 after slurry application.
Experimental design and sampling scheme of mesocosms. Please note that I, II, III represent different sampling plots at the site of soil origin. Resin bags for 15N analysis in leached NH4+ and NO3− were installed under the six mesocosms that were harvested at the end of the experiment. These mesocosms were also used for measurements of gaseous losses of N2O and N2 using static chambers over the entire time span of the experiment
15N labeled cattle slurry
The 15N labeling of the mesocosms was conducted via the application of 15N enriched liquid cattle slurry. The fresh slurry originated from a local farmer and was analyzed for N compounds by a commercial laboratory (Raiffeisen-Laborservice, Ormont, Germany). The analysis of N compounds was conducted following international standards for N analysis. Total nitrogen (TN) and dissolved N was determined by elemental analysis as outlined in DIN ISO 13878 (DIN 1998). Organic N was calculated based on the difference between total and dissolved N. The slurry had a pH of 7.9 and was characterized by a low background N content of 1.4 g N kg−1 FW (69.7 g N kg−1 DWa), with 0.4 g N kg−1 FW consisting of organic N and 1 g N kg−1 FW consisting of dissolved N, i.e., NH4+-N and urea-N. This low N slurry was 15N-labeled by adding 2.2 g urea-N g−1 FW and 2.2 g NH4+-N g−1 FW at 99 atom% 15N enrichment. For this, we generally followed the 15N slurry labeling procedure described in detail by Schlingmann et al. (2020), but used higher 15N addition rates to achieve the required high 15N tracer enrichments for the detection of 15N2 formation through denitrification (Friedl et al. 2020). Immediately prior to fertilization of the mesocosms, the 15N tracer was added to the slurry in a glass bottle and vigorously mixed. Following the regional standard farming practice of surface spreading, we then applied the slurry at a rate of 1.8 m3 ha−1 which translates to 40 ml for each mesocosm (Schlingmann et al. 2020). This equaled an N addition of 97.2 kg N ha−1, strongly dominated by plant-available N (urea- and NH4+-N). Through this procedure, 175.9 mg 15N in excess of natural abundance was added per mesocosm (79 atom % 15N enrichment). One hour following slurry application, we simulated a precipitation event of 30 mm, applied in 6 doses within 3 h.
Mesocosm harvest, soil and plant sampling
We followed the mesocosm sampling scheme described in detail by Schlingmann et al. (2020) and Schreiber et al. (2023, see also supplementary information), with the complete harvest being conducted within one day. In short, the soil column was pushed out of the stainless-steel core with a manual hydraulic device (Schreiber et al. 2023). Aboveground biomass was then sampled by cutting the turf directly above the soil with scissors. Subsequently, the soil column was cut into 3 pieces with a saw: 0–4 cm, 4–10 cm, 10–25 cm depth. Total soil of each layer was homogenized by hand in a bucket for at least 10 min. Roots were hand-picked and washed with tap water (Schreiber et al. 2023). Soil, AGB and BGB samples were dried at 55° C to a constant weight directly after sample preparation. Subsequently, samples were homogenized again, and a representative subsample was ground to a fine powder with a ball mill (Retsch Schwingmühle MM2, Haan, Germany) and stored until analysis in a desiccator over silica gel. The 15N enrichment in total soil N, AGB and BGB samples as well as the respective TN concentration was measured by elemental analysis coupled to isotope ratio mass spectrometry (Yankelzon et al. 2024a, b). For the assessment of inorganic N, fresh soil samples underwent an extraction process using 0.5 M K2SO4, maintaining a 1:2 ratio of soil weight to extract solution volume (Dannenmann et al. 2016). Following extraction, the samples were transferred into Falcon tubes and stored in a freezer for subsequent analysis. Concentrations of dissolved NH4+-N and NO3−-N were measured colorimetrically using a microplate spectrometer (BioTek Instruments, Inc.), following the methods described by Kempers and Zweers (1986) and Pai et al. (2021). Soil extracts were also analyzed for DOC concentrations (Multi N/C 3100, Analytik Jena, Germany) according to Dannenmann et al. (2016), 15N enrichment in NH4+ and NO3− was determined by sequential diffusion on acid filter traps, which were then analyzed via elemental analysis (Flash EA, Thermo Scientific, Waltham, MA, USA) coupled to isotope ratio mass spectrometry (IRMS) (Delta PlusXP, Thermo Scientific, Waltham, MA, USA) as described in detail by Schreiber et al. (2023). Gravimetric soil water content was determined by drying the fresh soil in an oven (105 °C) for over 24 h. The latter was used to calculate water-filled pore space (WFPS), thereby considering the respective mesocosm-layer specific bulk density, and a particle density of 2.65 g cm−3. We conducted additional two samplings for the determination of WFPS using a 3 cm thick corer on days 7 and 14.
Nitrogen leaching
Nitrogen leaching rates were measured with the six mesocosms that were harvested at the end of the experiment (Fig. 1). The leaching of mineral N was determined from the accumulation of NH4+- and NO3−- N and -15N on resin bags (Dannenmann et al. 2018). For this purpose, bags containing ion‐exchange resins (45 g of Amberlite, IR 120 [Na+‐ion exchanger resin] and 45 g Dowex 1 × 8 [Cl−‐ion exchanger resin]) were placed under six mesocosms and harvested at the end of the experiment. Resin bags were brought to the laboratory facilities of KIT-IMK-IFU in Garmisch-Partenkirchen and extracted with 1 M NaCl solution. The extracts were analyzed for NH4−-N and NO3−-N concentrations and 15N-enrichment in NH4+ and NO3− using the SPINMAS technique (Stange et al. 2007). The measurements were carried out in an automated sample preparatory (SPIN unit; InProcess, Bremen, Germany) coupled with a mass spectrometer (GAM 400; InProcess, Bremen, Germany).
Emissions of N2O and N2
Emissions of N2O and N2 were measured using the manual static chamber technique on the six mesocosms harvested on day 29. The dark steel chambers covered the entire mesocosms and had a height of 15.5 cm (see Unteregelsbacher et al. 2013 for further details). For quantification of N2O emissions, the sampling frequency was daily for the first two weeks post-fertilization. This frequency was then reduced to three measurements per week from the third week onwards. The chamber closure time was 120 min. Gas samples were taken between 10–12 am at 0, 30, 60 and 120 min after chamber closure and then transferred into pre-evacuated 12 ml glass vials with a double wadded Teflon/silicon septa cap (Labco, UK) and analyzed for N2O concentrations using gas chromatograph connected to an autosampler (SRI 8610C, SRI Instruments, Torrance, USA), following the method described by Rehschuh et al. (2019). Nitrous oxide fluxes were calculated based on the slope of linear increase in N2O concentration over time, thereby considering air temperature and atmospheric pressure (Rehschuh et al. 2019). Quality control involved determining the minimum detectable concentrations. This was done by multiplying the standard deviation of ambient air N2O levels by the t-value for a 95% confidence interval. These concentrations were then translated into fluxes, setting a threshold below 0.4 g N ha−1 day−1.
The 15NGF method was applied to quantify N2 losses daily for the first 7 days following fertilization, and then every two to four days until day 29. For this, additional vials were filled simultaneously with the N2O sampling, at 0, 30 and 120 min after chamber closure. These vials were analyzed for N2O and N2 and their respective isotopologues ((15N14N, 15N15N; [14N15N16O + 15N14N16O] and 15N15N16O) using an isotope ratio mass spectrometer (IRMS) (Sercon Limited, 20–20, UK) linked to a Sercon Cryoprep trace gas concentration system.
Emissions of N2 were calculated using the 15NGF method (Friedl et al. 2020). Briefly, measurements of the ion currents (I) via IRMS at the mass-to-charge ratio (m/z) 44, 45, and 46 allowed the molecular ratios 45R (45I/44I) and 46R (46I/44I) to be calculated for N2O. The 15N enrichment of the soil NO3− pool undergoing denitrification (aD) (Fig. S1) was estimated using 45R and 46R. The I at m/z 28, 29 and 30 gave 29R (28I/29I) and 30R (28I/30I) for N2, and the change in the respective ratios during chamber closure was expressed as Δ29R and Δ30R. Assuming that N2O derived from denitrification, and N2 were produced from the same NO3− pool, fluxes of N2 were estimated using Δ30R and aD (Mulvaney 1984; Stevens and Laughlin 2001), corrected for air temperature, and air pressure and expressed in g ha−1 day−1. The precision of the IMRS determined prior analysis at 95% confidence intervals (n = 10) was 6.16 × 10–7 and 4.60 × 10–7 for 29R and Δ30R, respectively. The excess 15N enrichment of N2O was used to calculate its fertilizer-derived fraction as outlined in Takeda et al. (2022). The measured %15N excess in N2O was corrected to account for the N2O existing in the headspace.
Calculation of 15N recovery in plant and soil N, and leached N
The excess 15N amount (mg) in all investigated N pools was calculated using the following equation.
where Npool is the amount of N [mg N mesocosm−1] in the plant or depth-specific soil N pool and 15NL is the enrichment (atom% 15N) of the respective N pool. We used 0.3663% as the natural abundance of 15N; errors induced by possible slight variations of 15N natural abundance were negligible due to the high enrichment obtained from the very large 15N slurry labeling. Dividing 15N excess amount in the analyzed pools by the cumulative 15N excess addition through slurry fertilization at the sampling time (175.87 mg 15N excess per mesocosm) revealed the 15N excess recovery, expressed as a percentage.
Fertilizer N fates and balance
The calculation of fertilizer N balances followed the approach described by Yankelzon et al. (2024a, this issue). Specifically, N flows from fertilizer into total soil N, into leached N, as well as into plant AGB and BGB N were calculated by dividing the respective 15N excess recovery rate (% of applied fertilizer 15N excess) by 100 and multiplying with the amount of total added fertilizer N (97.2 kg N ha−1). This total slurry N amount also includes unlabeled polymeric organic N of 7 kg N ha−1, which might behave differently compared to the plant available N which was 15N labeled. Unrecovered 15N was assumed to equal total gaseous N losses. Subtracting measured N2O and fertilizer-derived N2 losses then revealed NH3 emissions, assuming negligible NO emissions as confirmed previously for similar grassland mesocosms (Zistl-Schlingmann et al. 2019).
Statistical analysis
There were six replicate mesocosms per sampling date. Statistical analyses were performed in R version 4.1.3 with a 5% significance level. Before applying parametric tests, it was assured that data were normally distributed with Shapiro–Wilk tests or that variance was homogenous with Levene’s tests. Non-normally distributed data were log-transformed. We performed ANOVAs including post-hoc Tukey analyses correcting for multiple testing.
Results
Meteorological data
Following the slurry application on June 22, we directly simulated rainfall of 30 mm (Fig. 2A). There was no natural precipitation over three days after fertilization. However, in the following three weeks, precipitation was observed during 13 days, with the daily sum even exceeding 40 mm on June 28th and July 10th. Consequently, this resulted in high values of WFPS up to 100% in the deepest soil layer, with WFPS generally increasing with soil depth (Fig. 2B). In the very wet first 10 days of the experiment, also topsoil WFPS (0–4 cm and 4–10 cm) was high (range 60–80%). In all soil layers, soil moisture reached its highest values on day 8, induced by a daily precipitation of > 40 mm on day 7 (Fig. 2B). After these wet first 10 days, WFPS in all depths decreased until day 17, but only WFPS in 0–4 cm declined to ca 40% WFPS, while it did not drop below 60% WFPS in 4–10 cm (Fig. 2B). The presumable increase in WFPS after the second large precipitation event > 40 mm on June 10 was not captured by the sampling design (Fig. 2B). Daily mean air temperatures were quite variable during the course of the experiment and oscillated between ca 12 °C and 20 °C.
Daily precipitation and mean air temperature during the experiment (A); soil water-filled pore space (WFPS) in the three sampled soil layers (B); N2 emissions (C); and N2O emissions (D). Note that in (C and D) both total N emissions and fertilizer-derived (FD) emissions are provided. Furthermore, for N2 two interpolation techniques were used (linear interpolation between actual measurements; and a more conservative interpolation using N2 emissions of half of the method detection limit for all days with no measurements or measurements with no detectable N2 fluxes. N2O cumulative fluxes were based on linear interpolation. Slurry application (SA) and harvest times (H1-H5) are indicated by dotted lines. The error bars depict the standard errors derived from the means of six replicates
Emissions of N2 and N2O
The highest N2 emissions were measured immediately after fertilizer application, totaling 1.8 kg N ha−1 day−1, thereof 1.7 kg N ha−1 day−1 derived from fertilizer (Fig. 2C). These emissions quickly declined to values close to zero within 3 days, and slightly increased again after the large precipitation event on day 7 to form a smaller second peak of N2 emissions (Fig. 2B, C). This smaller peak slowly declined afterward, thereby following the dynamics of WFPS (Fig. 2B, C). The sampling on day 21 also revealed relatively high N2 emissions, which occurred two days after the second heavy precipitation event (Fig. 2A, C). Cumulative total N2 emissions were 4.4 kg N ha−1 when linear interpolation between measurement days was applied, and 3.1 kg N ha−1 when N2 fluxes at days with no measurements or undetectable fluxes were set to 50% of the method detection limit (conservative estimate). For fertilizer-derived N2 emissions, these values amounted to 3.3 and 2.7 kg N ha−1, respectively.
Nitrous oxide emission followed a generally similar pattern compared to N2 emission, but with a slower decline of the emissions (Fig. 2D). The highest emission, 27 g N ha−1 day−1 (24 g N ha−1 from fertilizer-derived N2O), was observed on the day of fertilization, followed by a subsequent decline to low background fluxes approximately within one week. Subsequently, only very minor changes in N2O fluxes were observed. The cumulative N2O fluxes totaled 0.1 kg N ha−1, of which 0.06 kg N ha−1 were fertilizer-derived. The N2O:(N2O + N2) emission ratio (RN2O) was well below 0.1 throughout the course of the measurements, except for days 3–6, when N2 emissions almost had disappeared, but minor N2O emissions still were present (Fig. S2). The overall RN2O, however, was very low, at 0.03.
Soil ammonium, nitrate and dissolved organic carbon
One day after fertilization, we found 47 kg NH4+-N in the soil of the mesocosms, thereof 42 kg NH4+-N in 0–4 cm depth (Fig. 3). Nitrate–N amounted to 35 kg N ha−1 and was more evenly spread across the three soil layers. Accordingly, the sum of mineral N found in all soil layers sampled (82 kg N ha−1) was close to the total added fertilizer N of 97 kg ha−1, of which 90 kg N ha−1 was in dissolved form (mainly urea and NH4+). On day 3 after fertilization, we still found a total of 82 kg N ha−1 of mineral N in the soil (Fig. 3), with NH4+ contributing only 10 kg N ha−1 and NO3− having doubled to 72 kg N ha−1 (Fig. 2). On days 10 and 17 after fertilization, the mineral N stocks in the soil had decreased by almost one order of magnitude to about 15 kg N ha−1, and further decreased to about 10 kg N ha−1 on day 29 at the end of the experiment (Fig. 3), with NO3− N always dominating the mineral N stocks during this time. Dissolved organic carbon (DOC) stocks dropped between days 1 and 3 and then remained unchanged (Fig. S4). The DOC: NO3− ratios and in particular the DOC: dissolved mineral N ratios in the first 3 days of the experiment were lowest in the top 0–4 cm (Fig. S5, S6). Specifically, DOC: dissolved mineral N ratios in the top 4 cm were as low as two on day 1 and only increased to about four (day 3) and five (day 10) (Fig. S6).
15N fertilizer recovery
One day after fertilizer application, we recovered 91 ± 5% of the added 15N-labeled fertilizer in the plant-soil system, indicating an initial gaseous loss of 9% during the first day (Fig. 4). The total 15N recovery decreased to a value of 78 ± 3% by day 3, but then did not change significantly until the end of the experiment, when the recovery was still 75 ± 2% (Fig. 4). Considering the negligible cumulative 15N recovery in leached N of 0.2% at the end of the experiment, this implies that the losses of about 25% were mainly via gaseous pathways (Fig. 4).
Recovery of fertilizer 15N excess in aboveground (AGB) and belowground (BGB) plant biomass, and in soil. Unrecovered 15N was assumed to equal total gaseous N losses (NH3, N2O, N2). Leaching (0.2% recovery only) was measured only for the last sampling on day 29. The error bars illustrate the standard errors obtained from averaging six replicates
At day 1, we already recovered 15% of the tracer in the roots (Fig. 4). This 15N recovery in the roots did not significantly change in the further course of the experiment (Fig. 4). With on average 90% relative contribution, roots in 0–4 cm depth were most important for 15N excess recovery, while roots in 4–10 cm depth (7% contribution) and in 10–25 cm depth (3%) only contributed to a minor extent to the tracer recovery (data not shown). With only 4% we recovered almost four times less 15N tracer in AGB compared to roots one day after the application of 15N fertilizer. However, the 15N recovery in AGB quickly increased to reach its maximum of 35% at day 17 with no further increase to the end of the experiment (Fig. 4). At days 17 and 29, about half of the 15N tracer was recovered in AGB and BGB.
We recovered almost 75% of fertilizer 15N excess in soil, one day after application, mostly in 4–10 cm, and to a much smaller extent also in 4–10 and 10–25 cm depth (Fig. 4). At day 3, 15N recovery in NO3− exceeded 15N recovery in NH4+ by already more than a factor of six (Fig. S3). In the following days and weeks, 15N recovery decreased in all soil layers, but particularly in 0–4 cm depth, until day 17 to about 25% of the applied 15N excess (Fig. 4). This equaled the increasing 15N excess recovery in plant biomass. From day 17 to day 29, the 15N excess recovery in soil did not significantly change, as was also observed for 15N recovery in plant biomass. The 15N recovery balance over time indicated that there were soil-born gaseous 15N losses between days 1 and 3 (Fig. 4). However, subsequent decreases in soil 15N recovery were matched by parallel increases in AGB 15N recovery, while no significant increase in unrecovered 15N excess (i.e., gaseous losses) was observed (Fig. 4).
Aboveground biomass N accumulation and origin
The AGB increased from 1.6 ± 0.3 t DM ha−1 the day after fertilization to 4.9 ± 0.4 t DM ha−1 on day 10, but did not show further significant changes between day 10 and day 29 (Table 1). Aboveground plant N increased from initially 24.6 ± 4.0 kg N ha−1 on day 1 after fertilization to reach its maximum of very large 100.4 ± 5.6 kg N ha−1 (equaling total fertilizer N without fertilizer N losses) at day 17 after fertilization, with no further increase being observed until day 29 (Table 1). Already one day after fertilization, 3.8 ± 0.7 kg N ha−1 or 15.3% of total AGB—N was derived from fertilizer. At day 3, this fertilizer-derived N in AGB amount was tripled, and reached its maximum of 33.6 ± 2.5 kg N ha−1 17 days after fertilization. Still, N from recent fertilizer application was of minor importance compared to other plant N sources, with fertilizer N contribution to AGB-N of 13–15% in the first three days and 32–34% during the later sampling times (Table 1). Fertilizer-derived N in total BGB was as high as 14 kg N ha−1 one day after slurry application, but then did not further increase (Table 1). Consequently, after 10 days, there was always more fertilizer-N in AGB compared to BGB.
Fertilizer N balance after one month
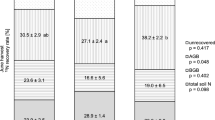
Despite the aforementioned low contribution of fertilizer N to total N in harvested AGB (less than 1/3), plant N uptake accounted for about half of the fertilizer N after one month, with 31% of fertilizer being allocated to AGB and 18% to BGB (Table 2). Only about 25% of fertilizer N was still recovered in soil, while about 25% was subject to N losses (Table 2). Among the directly measured losses, fertilizer-derived N2 emissions accounted for 3.4% (3.3 kg N ha−1), followed by leached fertilizer N (0.2 kg N ha−1 or 0.2%) and N2O emissions (0.06 kg N ha−1 or 0.06%) (Table 2). Based on a previous study (Zistl-Schlingmann et al. 2019), nitric oxide (NO) emissions in this soil are negligible, also after fertilization. Therefore, the remaining losses of 20.7 kg N ha−1 or 21.3% are assumed to be attributed to NH3 emissions.
Discussion
Dinitrogen emissions from slurry-fertilized montane grassland
This study is one of the few reports of direct in situ measurements of N2 emissions from grasslands. Based on the applied 15NGF method, we constrained total N2 emissions after the application of 97.2 kg of slurry-N (thereof 90 kg in form of NH4+ and urea-N) to 3.1 to 4.4 kg N ha−1 depending on the interpolation approach, with 2.7 to 3.3 kg N ha−1 being derived from the applied fertilizer. The very low N2O emissions of 0.1 kg N2O-N ha−1 observed from the studied mesocosms are well in line with earlier studies (Unteregelsbacher et al. 2013; Zistl-Schlingmann et al. 2019) and result in an extraordinarily low cumulative N2O/(N2 + N2O) ratio (RN2O) of 0.03. This is related to the high pH values in the soil around 7 which promotes high N2O reductase activity (Chen et al. 2015; Wu et al. 2020), thereby reducing N2O to N2 in the terminal step of denitrification. Furthermore, limited diffusion capacity induced by the clayey texture and high precipitation-induced WFPS as well as high O2 consumption through respiration, will also promote full denitrification to the end product N2 (Butterbach-Bahl et al. 2013; Friedl et al. 2022).
It is noteworthy that low RN2O was also observed during the first three days after fertilization, when high NO3− concentrations coincided with WFPS of 60–70% in the topsoil (Fig. 2). Given the ecology and stoichiometry of denitrification, these conditions should have limited the use of N2O as an electron acceptor, resulting in higher RN2O and significant N2O emissions. The absence of significant N2O emissions further supports the dominant role of pH in regulating denitrification stoichiometry in calcareous soils (Dannenmann et al. 2008; Liu et al. 2010). Still, high NO3− concentrations might have contributed to the increasing RN2O between days 3 and 6 (Fig. S2), which however was observed when N gas emissions had largely decreased. Taken together, this suggests that the addition of N-rich slurry hardly leads to increased N2O emissions from prealpine grassland soils.
Despite the application of very N-rich manure to soil that supports high total denitrification and N2 emission rates, the N2 emissions reported in this study were lower than in most of the few other available grassland studies. For example, McGeough et al. (2012) applied 65 kg NH4+15NO3− to grassland in Northern Ireland and reported emissions of 16.5 kg N2-N ha−1 (RN2O = 0.22) and 10.5 kg N2-N ha−1 (RN2O = 0.26) when a nitrification inhibitor was added. The latter study alternatively also used slurry instead of mineral N fertilizer, amended with 15NO3− (also 65 kg N ha−1) and then measured associated N2 emissions of 13.5—18.7 kg N ha−1 (RN2O 0.31 – 0.36). Buchen et al. (2016) assessed N2 emissions from grassland in Germany as affected by renewal and conversion to maize cropping. These authors reported, after the application of 80 kg 15NO3− ha−1, N2 emissions of 21 kg N ha−1 (old grassland), 12.7 kg N ha−1 (after grassland renewal) and 40 kg N ha−1 (after conversion to maize cropping), i.e., several fold larger N2 emissions than in our study, while the mean RN2O of 0.03 was comparable. Friedl et al. (2017) added only 37 kg 15N-labeled urea-N ha−1 to irrigated perennial dairy pastures in Australia for in situ quantification of denitrification fluxes using the 15NGF method. Despite lower N addition, they found similar N2 emission rates like in the present study, ranging between 1.1—3.9 kg N ha−1 depending on soil type and addition of nitrification inhibitors, while N2O emissions with 0.03 to 0.16 kg N ha−1 were also very low. Finally, Zistl-Schlingmann et al. (2019), using similar soil–plant mesocosms as in this study, revealed very high N2 emissions with a different laboratory incubation method, i.e., the He soil core technique. In this study, 51 kg N ha−1 was added as unmodified cattle slurry, and N2 emissions with 16–21 kg N ha−1 dominated fertilizer N losses, followed by NH3 (3.5 kg N ha−1), N2O (0.2–0.5 kg N ha−1) and NO emissions (0–0.2 kg N ha−1). Hence in the study of Zistl-Schlingmann et al. (2019), N2 emissions were about 5–6 times larger than measured in this study, while RN2O ranging from 0.01 to 0.04 was very similar. The latter study probably overestimated denitrification because the dark incubation prevented plant N uptake so that more NH4+ and NO3− were available for denitrification. This view is strongly supported by our study, as we found uptake of fertilizer N into AGB and BGB in the first 3 days of 33 kg N ha−1, i.e., one order of magnitude larger than denitrification N losses. It seems very plausible that denitrification would have been significantly larger without this large plant N uptake. This suggests that incubation systems based on the He soil core technique should be built with transparent chambers and light sources to allow for plant-soil-interactions such as competition for N, despite this strongly increases the engineering efforts involved in building such extremely gastight systems operating with He/O2 atmosphere (see Yankelzon et al. 2024b, this issue). Schlingmann et al. (2020) conducted a field lysimeter experiment with the same soil using two applications of 15N labeled cattle slurry of together 76 kg N ha−1 and reported total seasonal gaseous N losses of 37 kg N ha−1 based on unrecovered fertilizer 15N. Referencing the relative contribution of N2, N2O, NO and NH3 to gaseous N losses obtained in the laboratory He soil core study of Zistl-Schlingmann et al. (2019) to the unrecovered slurry 15N of the lysimeter field study would result in a slightly more conservative seasonal estimate of N2 emissions of 28–32 kg N2-N ha−1 season−1 or 14–16 kg N2-N ha−1 for one fertilization event.
Nevertheless, the relatively low N2 emissions measured in this study under in situ conditions are surprising because the soil has a high pH, NO3− concentration and WFPS, all of which support ideal conditions for denitrification. However, denitrification as a heterotrophic process is also highly dependent on labile C sources. Therefore, low denitrification rates under high NO3− availability and WFPS in this study could be due to a lack of labile C, which is confirmed by the very low DOC: mineral N ratios in the topsoil (Fig. S6). Furthermore, the huge conversion of NH4+ to NO3− of about 36 kg N ha−1 between day 1 and day 3, suggesting net autotrophic nitrification of 18 kg N ha−1 day−1 also supports a lack of available C. It has already been shown that autotrophic nitrification dominates gross nitrification (Wang et al. 2014), and these net rates are very high given a reported cumulative growing season gross nitrification of only ca 200–250 kg N ha−1 season−1 in the studied soil (Wang et al. 2016). Because autotrophic nitrification is a competitive inferior process in NH4+ partitioning compared to the energetically superior heterotrophic microbial NH4+ immobilization, it only occurs at high rates under limited labile C availability (Butterbach-Bahl and Dannenmann 2012). Therefore, the very high ammoniacal N application could have initially triggered a very short-lived growth of free-living heterotrophic microorganisms until the labile C dissolved in the soil was exhausted, which could then also have impaired denitrification, thus explaining the rapidly declining N2 emissions. This view is also supported by the relatively low soil N stabilization in this study compared to a previous study, which found a much larger soil 15N recovery driven by microbial immobilization (Zistl-Schlingmann et al. 2020), despite this comparison is only possible for a period of 1 month.
However, also method-inherent shortcomings of the 15NGF method might lead to an underestimation of N2 emissions. While the He soil core technique quantifies all N2 emissions independent of their origin in the soil profile and independent of the source process and probably overestimates emissions from plant-soil systems in dark chambers as outlined above, the 15NGF technique might systematically underestimate N2 emissions. This is mainly because of heterogeneous tracer application, and due to 15N2 subsoil diffusion and storage (Arah 1992; Friedl et al. 2020; Micucci et al. 2023; Vanden Heuvel et al. 1988; Well et al. 2019). With a 15N tracer recovery of 56% observed one day after the addition in the 0–4 cm soil depth and 17% in the 4–25 cm soil depth, our study highlights the difficulty of homogeneously labeling vertical soil profiles (Fig. 3). Only N2 from unlabeled N sources which are well mixed with labeled N will be detected as 15N2 flux in the chamber headspace. It thus appears possible that we missed N2 emissions from deeper soil layers, where there was not sufficient mixing with the 15N tracer. However, this should be of little relevance for fertilizer-derived N2 emissions. The underestimation due to heterogenous 15N labeling has been estimated to be around 25% (Arah 1992; Vanden Heuvel et al. 1988), and an even larger underestimation due to subsoil diffusion of up to 70% was reported by Well et al. (2019). Consequently, N2 emissions could be up to twice as high as suggested by our direct measurements using the 15NGF method, i.e., about 6 kg N for a single manure application, which would be closer to the above estimate of 14–16 kg derived from the combination of laboratory measurements with the He technique and field 15N balance studies of Zistl-Schlingmann et al. (2019) and Schlingmann et al. (2020). Also considering a possible underestimation of N2 emission, which would result in an overestimation of NH3 emissions due to our mass balance approach, our data still indicate that N2 emissions remained clearly smaller than NH3 emissions, but still represent the second most important gaseous N loss pathway.
Dynamics of fertilizer partitioning in the plant-soil system
The high temporal resolution of this 15N tracing study allowed unprecedented detailed insight into the dynamics of fertilizer N fluxes. We distinguished several mechanisms and processes occurring on different time scales: (1) fertilizer input via leaching resulted in increased mineral N concentrations mainly in the top 4 cm, although N concentrations also increased somewhat in deeper soil layers (immediately after fertilization); (2) a phase marked by the rapid conversion of ammoniacal N into volatilized NH3, accompanied by strong nitrification and subsequent denitrification (until day 3); (3) plant N uptake of fertilizer N mainly from the top 0–4 cm of soil (until day 17), especially in the form of NO3− after day 3; and (4) no further detectable fertilizer N dynamics between day 17–29.
Contrary to our expectations, plant N uptake of applied manure N, especially into roots, occurred at high rates as early as 1 day after fertilization and quantitatively exceeded denitrification N losses by an order of magnitude after 3 days (Fig. 3). Such high initial fertilizer N uptake by plants may be facilitated by the arbuscular mycorrhizal fungal (AMF) community, which is abundant in the extensively managed grassland of this study (Andrade-Linares et al. 2023). It should be noted that due to our sampling strategy, part of the AMF hyphae would be sampled as soil. Therefore, even more N taken up by mycorrhiza would have to be attributed to roots. The maximum plant N uptake of 100 kg N ha−1 reached on day 17 (of which 34 kg derived from fertilizer N) is about twice as high as in a previous study at the same site (Schlingmann et al. 2020), but similar to the N output per harvest as observed for grasslands at higher altitudes with higher SOC content (Zistl-Schlingmann et al. 2020). Despite the huge total plant N uptake, only about 1/3 of plant N was derived from recent fertilizer, leaving 2/3 to be acquired from other N sources such as biological N2 fixation, atmospheric deposition, and likely dominant mineralization and nitrification of soil organic N (Wang et al. 2016; Zistl-Schlingmann et al. 2020). The contribution of recent fertilizer N to plant N was even much lower (3–11%) in previous studies in comparable grasslands (Schlingmann et al. 2020).
Grassland harvesting is an important N export pathway, with exported N originating mainly from non-fertilizer N. Therefore, grassland harvesting can be a key driver of soil N mining. In this context, it is interesting to see if the application of N-rich slurry, as in our study, may prevent soil N mining. Subtracting 25 kg N (total N gaseous losses) and 0.2 kg N (leaching losses) as well as 95.6 (plant N uptake) from the N input of 97.2 kg N ha−1 results in a deficit of 24 kg N ha−1 calculated for the one month period of this study. Considering atmospheric N deposition and biological N2 fixation, which are estimated to be 33 and 10 kg N ha−1 year−1, respectively (Schlingmann et al. 2020) suggests a neutral N balance for two cuts and a deficit starting with three or more cuts based on our study.
Previous studies conducted at comparable grassland sites, where lower rates of cattle slurry N application were employed, reported larger N deficits ranging between 50 kg N ha−1 year−1 (2–3 fertilization/cutting cycles) and 100 kg N ha−1 year−1 (approximately 4–5 fertilization/cutting cycles). These deficits were further amplified under experimentally simulated climate change conditions (Schlingmann et al. 2020; Zistl-Schlingmann et al. 2020). Such SON mining is closely related to SOC mining as demonstrated by Wang et al. (2021) for comparable grassland systems. In this context, N mining can be mitigated either by reduced plant N output and/or when fertilizer N losses are reduced. The fertilizer N losses in this study, at 25% of the applied N, are relatively small and consistent with the findings of Schreiber et al. (2023). In contrast, Schlingmann et al. (2020) and Zistl-Schlingmann et al. (2020) reported slurry N losses ranging between 40–60% of applied N, despite all three studies adhering to similar slurry application timings. Given this large variability of slurry N losses, further studies should be targeted to identify the management, soil and climatic factors that govern slurry N fates. Particularly, improved slurry application techniques such as open slot injection or slurry acidification (Buchen-Tschiskale et al. 2023; Emmerling et al. 2020; Schreiber et al. 2023) are expected to reduce grassland N losses and SON mining.
Conclusion
The present study constrains both N2O and N2 emissions from organic matter-rich pre-alpine grasslands after manure application based on direct in situ 15N gas flux measurements. However, it also highlights the associated uncertainties due to persistent methodological problems. Our results suggest that N-rich slurry application does not lead to large N2O losses from organic matter-rich pre-alpine grassland soils with neutral pH, at least when fertilization is followed by precipitation events. This is partly due to the reduction of N2O to N2 and partly due to the rapid uptake of N by plants, which exceeds total denitrification by an order of magnitude already 3 days after fertilization. As a result, N2 emissions accounted for only 3% of the added fertilizer N. The high plant N uptake, together with microbial immobilization, appears to enhance the filter capacity of the soil, preventing N leaching despite high nitrification of ammoniacal N from the fertilizer and extensive precipitation, which generally favors N leaching. Thus, plants played a pivotal role in mitigating significant denitrification N losses and leaching, which underscores the importance of N fertilization timing adjusted to grass growth patterns to reduce N losses. However, our N mass balance estimates indicate that NH3 losses were up to 20% of the applied N, emerging as the predominant N loss pathway. Our findings also suggest that dark chamber incubations, typically used in He soil core approaches, may severely overestimate denitrification in such grasslands due to impaired plant N uptake. The high temporal resolution of fertilizer 15N partitioning data in the plant-soil microbial system provided in this study is excellent for comprehensive testing of biogeochemical models, which can ultimately be used to further explore sustainable grassland management options at the regional scale.
References
Almaraz M, Wong MY, Yang WH (2020) Looking back to look ahead: a vision for soil denitrification research. Ecol 101:e02917. https://doi.org/10.1002/ecy.2917
Andrade-Linares DR, Schwerdtner U, Schulz S, Dannenmann M, Spohn M, Baum C, Gasche R, Wiesmeier M, Garcia-Franco N, Schloter M (2023) Climate change and management intensity alter spatial distribution and abundance of P mineralizing bacteria and arbuscular mycorrhizal fungi in mountainous grassland soils. Soil Biol Biochem 186:109175. https://doi.org/10.1016/j.soilbio.2023.109175
Arah JRM (1992) New Formulae for Mass Spectrometric Analysis of Nitrous Oxide and Dinitrogen Emissions. Soil Sci Soc Am J 56:795–800. https://doi.org/10.2136/sssaj1992.03615995005600030020x
Bardgett RD, Wardle DA (2003) Herbivore-Mediated Linkages between Aboveground and Belowground Communities. Ecology 84:2258–2268 (http://www.jstor.org/stable/3450132and)
Buchen C, Lewicka-Szczebak D, Fuß R, Helfrich M, Flessa H, Well R (2016) Fluxes of N2 and N2O and contributing processes in summer after grassland renewal and grassland conversion to maize cropping on a Plaggic Anthrosol and a Histic Gleysol. Soil Biol Biochem 101:6–19. https://doi.org/10.1016/j.soilbio.2016.06.028
Buchen-Tschiskale C, Well R, Flessa H (2023) Tracing nitrogen transformations during spring development of winter wheat induced by 15N labeled cattle slurry applied with different techniques. Sci Total Environ 871:162061. https://doi.org/10.1016/j.scitotenv.2023.162061
Butterbach-Bahl K, Dannenmann M (2012) Soil carbon and nitrogen interactions and biosphere-atmosphere exchange of nitrous oxide and methane. In: Lal R, Lorenz K, Hüttl RF, Schneider BU, von Braun J (eds) Recarbonization of the Biosphere. Springer, Dordrecht, Netherlands, pp 429–443
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc B Biol Sci 368:20130122. https://doi.org/10.1098/rstb.2013.0122
Capriel P (2013) Trends in organic carbon and nitrogen contents in agricultural soils in Bavaria (South Germany) between 1986 and 2007. Eur J Soil Sci 64:445–454. https://doi.org/10.1111/ejss.12054
Cardenas LM, Bhogal A, Chadwick DR, McGeough K, Misselbrook T, Rees RM, Thorman RE, Watson CJ, Williams JR, Smith KA, Calvet S (2019) Nitrogen use efficiency and nitrous oxide emissions from five UK fertilised grasslands. Sci Total Environ 661:696–710. https://doi.org/10.1016/j.scitotenv.2019.01.082
Chang C, Entz T (1996) Nitrate Leaching Losses Under Repeated Cattle Feedlot Manure Applications in Southern Alberta. J Environ Qual 25:145–153. https://doi.org/10.2134/jeq1996.00472425002500010019x
Chen Z, Wang C, Gschwendtner S, Willibald G, Unteregelsbacher S, Lu H, Kolar A, Schloter M, Butterbach-Bahl K, Dannenmann M (2015) Relationships between denitrification gene expression, dissimilatory nitrate reduction to ammonium and nitrous oxide and dinitrogen production in montane grassland soils. Soil Biol Biochem 87:67–77. https://doi.org/10.1016/j.soilbio.2015.03.030
Christian DG, Poulton PR, Riche AB, Yates NE (1997) The recovery of 15N-labelled fertilizer applied to Miscanthus × giganteus. Biomass Bioenergy 12:21–24. https://doi.org/10.1016/S0961-9534(96)00060-8
Dannenmann M, Butterbach-Bahl K, Gasche R, Willibald G, Papen H (2008) Dinitrogen emissions and the N2:N2O emission ratio of a Rendzic Leptosol as influenced by pH and forest thinning. Soil Biol Biochem 40:2317–2323. https://doi.org/10.1016/j.soilbio.2008.05.009
Dannenmann M, Bimüller C, Gschwendtner S, Leberecht M, Tejedor J, Bilela S, Gasche R, Hanewinkel M, Baltensweiler A, Kögel-Knabner I, Polle A, Schloter M, Simon J, Rennenberg H (2016) Climate change impairs nitrogen cycling in European beech forests. PLoS ONE 11:e0158823. https://doi.org/10.1371/journal.pone.0158823
Dannenmann M, Díaz-Pinés E, Kitzler B, Karhu K, Tejedor J, Ambus P, Parra A, Sánchez-Martin L, Resco V, Ramírez DA, Povoas-Guimaraes L, Willibald G, Gasche R, Zechmeister-Boltenstern S, Kraus D, Castaldi S, Vallejo A, Rubio A, Moreno JM, Butterbach-Bahl K (2018) Postfire nitrogen balance of Mediterranean shrublands: Direct combustion losses versus gaseous and leaching losses from the postfire soil mineral nitrogen flush. Glob Change Biol 24:4505–4520. https://doi.org/10.1111/gcb.14388
DIN, I (1998) Bodenbeschaffenheit-Bestimmung des Gesamt-Stickstoffs durch trockene Verbrennung (Elementaranalyse), Beuth, Berlin, Wien, and Zurich. DIN ISO 13878. https://cir.nii.ac.jp/crid/1571698600104613504.bib?lang=en
Emmerling C, Krein A, Junk J (2020) Meta-Analysis of Strategies to Reduce NH3 Emissions from Slurries in European Agriculture and Consequences for Greenhouse Gas Emissions. Agronomy 10:1633. https://doi.org/10.3390/agronomy10111633
Fangueiro D, Surgy S, Napier V, Menaia J, Vasconcelos E, Coutinho J (2014) Impact of slurry management strategies on potential leaching of nutrients and pathogens in a sandy soil amended with cattle slurry. J Environ Manage 146:198–205. https://doi.org/10.1016/j.jenvman.2014.07.027
Friedl J, Scheer C, Rowlings DW, Mumford MT, Grace PR (2017) The nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) reduces N2 emissions from intensively managed pastures in subtropical Australia. Soil Biol Biochem 108:55–64. https://doi.org/10.1016/j.soilbio.2017.01.016
Friedl J, Cardenas LM, Clough TJ, Dannenmann M, Hu C, Scheer C (2020) Measuring denitrification and the N2O:(N2O + N2) emission ratio from terrestrial soils. Curr Opin Environ Sustain 47:61–71. https://doi.org/10.1016/j.cosust.2020.08.006
Friedl J, Deltedesco E, Keiblinger KM, Gorfer M, De Rosa D, Scheer C, Grace PR, Rowlings DW (2022) Amplitude and frequency of wetting and drying cycles drive N2 and N2O emissions from a subtropical pasture. Biol Fertil Soils 58:593–605. https://doi.org/10.1007/s00374-022-01646-9
Fu J, Gasche R, Wang N, Lu H, Butterbach-Bahl K, Kiese R (2017) Impacts of climate and management on water balance and nitrogen leaching from montane grassland soils of S-Germany. Environ Pollut 229:119–131. https://doi.org/10.1016/j.envpol.2017.05.071
Gao W, Fan C, Zhang W, Li N, Liu H, Chen M (2023) Heterotrophic nitrification of organic nitrogen in soils: process, regulation, and ecological significance. Biol Fertil Soils 59:261–274. https://doi.org/10.1007/s00374-023-01707-7
Garcia-Franco N, Wiesmeier M, Buness V, Berauer BJ, Schuchardt MA, Jentsch A, Schlingmann M, Andrade-Linares D, Wolf B, Kiese R, Dannenmann M, Kögel-Knabner I (2024) Rapid loss of organic carbon and soil structure in mountainous grassland topsoils induced by simulated climate change. Geoderma 442:116807. https://doi.org/10.1016/j.geoderma.2024.116807
Groffman PM (2012) Terrestrial denitrification: challenges and opportunities. Ecol Process 1:11. https://doi.org/10.1186/2192-1709-1-11
Harrison KA, Bol R, Bardgett RD (2007) Preferences for Different Nitrogen Forms by Coexisting Plant Species and Soil Microbes. Ecology 88:989–999 (http://www.jstor.org/stable/27651188)
Harrison KA, Bol R, Bardgett RD (2008) Do plant species with different growth strategies vary in their ability to compete with soil microbes for chemical forms of nitrogen? Soil Biol Biochem 40:228–237. https://doi.org/10.1016/j.soilbio.2007.08.004
Hart PBS, Rayner JH, Jenkinson DS (1986) Influence of pool substitution on the interpretation of fertilizer experiments with 15N. J Soil Sci 37:389–403. https://doi.org/10.1111/j.1365-2389.1986.tb00372.x
Kempers AJ, Zweers A (1986) Ammonium determination in soil extracts by the salicylate method. Commun Soil Sci Plant Anal 17:715–723. https://doi.org/10.1080/00103628609367745
Kiese R, Fersch B, Baessler C, Brosy C, Butterbach-Bahl K, Chwala C, Dannenmann M, Fu J, Gasche R, Grote R, Jahn C, Klatt J, Kunstmann H, Mauder M, Rödiger T, Smiatek G, Soltani M, Steinbrecher R, Völksch I, Schmid HP (2018) The TERENO Pre-Alpine Observatory: Integrating Meteorological, Hydrological, and Biogeochemical Measurements and Modeling. Vadose Zone J 17:1–17. https://doi.org/10.2136/vzj2018.03.0060
Laborde D, Mamun A, Martin W, Piñeiro V, Vos R (2021) Agricultural subsidies and global greenhouse gas emissions. Nat Commun 12:2601. https://doi.org/10.1038/s41467-021-22703-1
Liu B, Mørkved PT, Frostegård Å, Bakken LR (2010) Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol Ecol 72:407–417. https://doi.org/10.1111/j.1574-6941.2010.00856.x
Malique F, Wangari E, Andrade-Linares DR, Schloter M, Wolf B, Dannenmann M, Schulz S, Butterbach-Bahl K (2021) Effects of slurry acidification on soil N2O fluxes and denitrification. J Plant Nutr Soil Sci 184:696–708. https://doi.org/10.1002/jpln.202100095
McGeough KL, Laughlin RJ, Watson CJ, Müller C, Ernfors M, Cahalan E, Richards KG (2012) The effect of cattle slurry in combination with nitrate and the nitrification inhibitor dicyandiamide on in situ nitrous oxide and dinitrogen emissions. Biogeosciences 9:4909–4919. https://doi.org/10.5194/bg-9-4909-2012
Micucci G, Sgouridis F, McNamara NP, Krause S, Lynch I, Roos F, Well R, Ullah S (2023) The 15N-Gas flux method for quantifying denitrification in soil: Current progress and future directions. Soil Biol Biochem 184:109108. https://doi.org/10.1016/j.soilbio.2023.109108
Mulvaney RL (1984) Determination of 15N-Labeled Dinitrogen and Nitrous Oxide with Triple-Collector Mass Spectrometers. Soil Sci Soc Am J 48:690–692. https://doi.org/10.2136/sssaj1984.03615995004800030045x
Nyameasem JK, Zutz M, Kluß C, ten Huf M, Essich C, Buchen-Tschiskale C, Ruser R, Flessa H, Olfs H-W, Taube F, Reinsch T (2022) Impact of cattle slurry application methods on ammonia losses and grassland nitrogen use efficiency. Environ Pollut 315:120302. https://doi.org/10.1016/j.envpol.2022.120302
Pai SC, Su YT, Lu MC, Chou Y, Ho TY (2021) Determination of nitrate in natural waters by vanadium reduction and the Griess assay: Reassessment and optimization. ACS ES&T Water 1:1524–1532. https://doi.org/10.1021/acsestwater.1c00065
Rehschuh S, Fuchs M, Tejedor J, Schäfler-Schmid A, Magh R-K, Burzlaff T, Rennenberg H, Dannenmann M (2019) Admixing Fir to European Beech Forests Improves the Soil Greenhouse Gas Balance. Forests 10:213. https://doi.org/10.3390/f10030213
Rodhe L, Pell M, Yamulki S (2006) Nitrous oxide, methane and ammonia emissions following slurry spreading on grassland. Soil Use Manag 22:229–237. https://doi.org/10.1111/j.1475-2743.2006.00043.x
Rowlings DW, Scheer C, Liu S, Grace PR (2016) Annual nitrogen dynamics and urea fertilizer recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates. Agric Ecosyst Environ 216:216–225. https://doi.org/10.1016/j.agee.2015.09.025
Scheiner JD, Gutiérrez-Boem FH, Lavado RS (2002) Sunflower nitrogen requirement and 15N fertilizer recovery in Western Pampas, Argentina. Eur J Agron 17:73–79. https://doi.org/10.1016/S1161-0301(01)00147-2
Schlingmann M (2020) Effects of climate change and management on biogeochemical N turnover, slurry N use efficiency and associated pedosphere-atmosphere-hydrosphere exchange of N compounds in montane grasslands. Dissertation, Albert-Ludwigs-Universität
Schlingmann M, Tobler U, Berauer B, Garcia-Franco N, Wilfahrt P, Wiesmeier M, Jentsch A, Wolf B, Kiese R, Dannenmann M (2020) Intensive slurry management and climate change promote nitrogen mining from organic matter-rich montane grassland soils. Plant Soil 456:81–98. https://doi.org/10.1007/s11104-020-04697-9
Schreiber M, Bazaios E, Ströbel B, Wolf B, Ostler U, Gasche R, Schlingmann M, Kiese R, Dannenmann M (2023) Impacts of slurry acidification and injection on fertilizer nitrogen fates in grassland. Nutr Cycl Agroecosyst 125:171–186. https://doi.org/10.1007/s10705-022-10239-9
Schröder JJ, Schröder JJ, Jansen AG, Hilhorst GJ (2005) Long-term nitrogen supply from cattle slurry. Soil Use Manag 21:196–204. https://doi.org/10.1079/SUM2005306
Smith KA, Beckwith CP, Chalmers AG, Jackson DR (2002) Nitrate leaching following autumn and winter application of animal manures to grassland. Soil Use Manag 18:428–434. https://doi.org/10.1111/j.1475-2743.2002.tb00262.x
Stange C, Spott O, Apelt B, Russow R (2007) Automated and rapid online determination of 15N abundance and concentration of ammonium, nitrite, or nitrate in aqueous samples by the SPINMAS technique. Isot Environ Health Stud 43:227–236. https://doi.org/10.1080/10256010701550658
Stevens RJ, Laughlin RJ (2001) Lowering the detection limit for dinitrogen using the enrichment of nitrous oxide. Soil Biol Biochem 33:1287–1289. https://doi.org/10.1016/S0038-0717(01)00036-0
Sutton MA, Erisman JW, Dentener F, Möller D (2008) Ammonia in the environment: From ancient times to the present. Environ Pollut 156:583–604. https://doi.org/10.1016/j.envpol.2008.03.013
Takeda N, Friedl J, Kirkby R, Rowlings D, De Rosa D, Scheer C, Grace P (2022) Interaction between soil and fertiliser nitrogen drives plant nitrogen uptake and nitrous oxide (N2O) emissions in tropical sugarcane systems. Plant Soil 477:647–663. https://doi.org/10.1007/s11104-022-05458-6
Unteregelsbacher S, Gasche R, Lipp L, Sun W, Kreyling O, Geitlinger H, Kögel-Knabner I, Papen H, Kiese R, Schmid H-P, Dannenmann M (2013) Increased methane uptake but unchanged nitrous oxide flux in montane grasslands under simulated climate change conditions. Eur J Soil Sci 64:586–596. https://doi.org/10.1111/ejss.12092
Vanden Heuvel RM, Mulvaney RL, Hoeft RG (1988) Evaluation of Nitrogen-15 Tracer Techniques for Direct Measurement of Denitrification in Soil: II. Simulation Studies. Soil Sci Soc Am J 52:1322–1326. https://doi.org/10.2136/sssaj1988.03615995005200050021x
Wang C, Dannenmann M, Meier R, Butterbach-Bahl K (2014) Inhibitory and side effects of acetylene (C2H2) and sodium chlorate (NaClO3) on gross nitrification, gross ammonification and soil-atmosphere exchange of N2O and CH4 in acidic to neutral montane grassland soil. Eur J Soil Biol 65:7–14. https://doi.org/10.1016/j.ejsobi.2014.08.006
Wang C, Chen Z, Unteregelsbacher S, Lu H, Gschwendtner S, Gasche R, Kolar A, Schloter M, Kiese R, Butterbach-Bahl K, Dannenmann M (2016) Climate change amplifies gross nitrogen turnover in montane grasslands of Central Europe in both summer and winter seasons. Glob Change Biol 22:2963–2978. https://doi.org/10.1111/gcb.13353
Wang N, Xia L, Goodale CL, Butterbach-Bahl K, Kiese R (2021) Climate Change Can Accelerate Depletion of Montane Grassland C Stocks. Glob Biogeochem Cycles 35:e2020GB006792. https://doi.org/10.1029/2020GB006792
Well R, Maier M, Lewicka-Szczebak D, Köster J-R, Ruoss N (2019) Underestimation of denitrification rates from field application of the 15N gas flux method and its correction by gas diffusion modelling. Biogeosciences 16:2233–2246. https://doi.org/10.5194/bg-16-2233-2019
Wu X, Chen Z, Kiese R, Fu J, Gschwendter S, Schloter M, Liu C, Butterbach-Bahl K, Wolf B, Dannenmann M (2020) Dinitrogen (N2) pulse emissions during freeze-thaw cycles from montane grassland soil. Biol Fertil Soils 56:959–972. https://doi.org/10.1007/s00374-020-01476-7
Xu Y, He J, Cheng W, Xing X, Li L (2010) Natural 15N abundance in soils and plants in relation to N cycling in a rangeland in Inner Mongolia. J Plant Ecol 3:201–207. https://doi.org/10.1093/jpe/rtq023
Yankelzon I, Schilling L, Butterbach-Bahl K, Gasche R, Han J, Hartl L, Kepp J, Matson A, Ostler U, Scheer C, Schneider K, Tenspolde A, Well R, Wolf B, Wrage-Moennig N, Dannenmann M (2024a) Lysimeter-based full fertilizer 15N balances corroborate direct dinitrogen emission measurements using the 15N gas flow method. Biol Fertil Soils. https://doi.org/10.1007/s00374-024-01801-4
Yankelzon I, Willibald G, Dannenmann M, Malique F, Ostler U, Scheer C, Butterbach-Bahl K (2024b) A New Incubation System to Simultaneously Measure N2 as Well as N2O and CO2 Fluxes from Plant-Soil Mesocosms. Biol Fertil Soils. https://doi.org/10.1007/s00374-024-01809-w
Zistl-Schlingmann M, Feng J, Kiese R, Stephan R, Zuazo P, Willibald G, Wang C, Butterbach-Bahl K, Dannenmann M (2019) Dinitrogen emissions: an overlooked key component of the N balance of montane grasslands. Biogeochemistry 143:15–30. https://doi.org/10.1007/s10533-019-00547-8
Zistl-Schlingmann M, Kwatcho Kengdo S, Kiese R, Dannenmann M (2020) Management Intensity Controls Nitrogen-Use-Efficiency and Flows in Grasslands—A 15N Tracing Experiment. Agronomy 10:606. https://doi.org/10.3390/agronomy10040606
Acknowledgments
This work was funded by the German Federal Ministry of Education and Research (BMBF) in the SUSALPS project (Sustainable use of alpine and prealpine grassland soils in a changing climate, FKZ 031B0516A), embedded within the BonaRes initiative, and by the German Science Foundation (DFG) through the research unit DASIM. Further funding was obtained from the Helmholtz TERENO (Terrestrial Environmental Observatories) initiative. Johannes Friedl acknowledges support from the Austrian Science Fund through funding for the project ‘Sources matter - N2O production and consumption in grasslands’ (ESP 319-B).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dannenmann, M., Yankelzon, I., Wähling, S. et al. Fates of slurry-nitrogen applied to mountain grasslands: the importance of dinitrogen emissions versus plant N uptake. Biol Fertil Soils (2024). https://doi.org/10.1007/s00374-024-01826-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00374-024-01826-9