Abstract
Purpose
Before blastocyst development, embryos undergo morphological and metabolic changes crucial for their subsequent growth. This study aimed to investigate the relationship between morula compaction and blastocyst formation and the subsequent chromosomal status of the embryos.
Methods
This retrospective cohort study evaluated embryo development (n = 371) using time-lapse imaging; 94 blastocysts underwent preimplantation genetic testing for aneuploidy (PGT-A).
Results
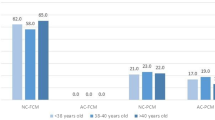
The embryos were classified as fully (Group 1, n = 194) or partially (Group 2, n = 177) compacted. Group 1 had significantly higher proportions of good- and average-quality blastocysts than Group 2 (21.6% vs. 3.4%, p = 0.001; 47.9% vs. 26.6%, p = 0.001, respectively). The time from the morula stage to the beginning and completion of compaction and blastocyst formation was significantly shorter in Group 1 than in Group 2 (78.6 vs. 82.4 h, p = 0.001; 87.0 vs. 92.2 h, p = 0.001; 100.2 vs. 103.7 h, p = 0.017, respectively). Group 1 embryos had larger surface areas than Group 2 embryos at various time points following blastocyst formation. Group 1 blastocysts had significantly higher average expansion rates than Group 2 blastocysts (653.6 vs. 499.2 μm2/h, p = 0.001). PGT-A revealed a higher proportion of euploid embryos in Group 1 than in Group 2 (47.2% vs. 36.6%, p = 0.303).
Conclusion
Time-lapse microscopy uncovered a positive relationship between compaction and blastocyst quality and its association with embryo ploidy. Hence, compaction evaluation should be prioritized before blastocyst selection for transfer or cryopreservation.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. For further inquiries, please direct them to the corresponding authors.
References
Nagy ZP, Shapiro D, Chang CC (2020) Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril 113:241–247. https://doi.org/10.1016/j.fertnstert.2019.12.009
Gardner DK, Meseguer M, Rubio C, Treff NR (2015) Diagnosis of human preimplantation embryo viability. Hum Reprod Update 21:727–747. https://doi.org/10.1093/humupd/dmu064
Hill MJ, Eubanks AE, Csokmay JM, Christy AY, Jahandideh S, DeCherney AH, Devine K, Levens ED, Connell MT (2020) Is transferring a lower-quality embryo with a good-quality blastocyst detrimental to the likelihood of live birth? Fertil Steril 114:338–345. https://doi.org/10.1016/j.fertnstert.2020.03.027
Park JK, Ahn SY, Seok SH, Chang EM, Kim JW, Kwak IP, Lee WS (2022) Does post-warming extended culture duration affect the clinical and obstetric outcomes of patients of advanced maternal age? A single-center study. J Korean Med Sci 37:e96. https://doi.org/10.3346/jkms.2022.37.e96
Huang TT, Huang DH, Ahn HJ, Arnett C, Huang CT (2019) Early blastocyst expansion in euploid and aneuploid human embryos: evidence for a non-invasive and quantitative marker for embryo selection. Reprod Biomed Online 39:27–39. https://doi.org/10.1016/j.rbmo.2019.01.010
Coticchio G, Borini A, Zacà C, Makrakis E, Sfontouris I (2022) Fertilization signatures as biomarkers of embryo quality. Hum Reprod 37:1704–1711. https://doi.org/10.1093/humrep/deac123
Venturas M, Yang X, Sakkas D, Needleman D (2023) Noninvasive metabolic profiling of cumulus cells, oocytes, and embryos via fluorescence lifetime imaging microscopy: a mini-review. Hum Reprod 38:799–810. https://doi.org/10.1093/humrep/dead063
Nguyen Q, Sommer S, Greene B, Wrenzycki C, Wagner U, Ziller V (2018) Effects of opening the incubator on morphokinetics in mouse embryos. Eur J Obstet Gynecol Reprod Biol 229:64–69. https://doi.org/10.1016/j.ejogrb.2018.08.003
Lundin K, Park H (2020) Time-lapse technology for embryo culture and selection. Ups J Med Sci 125:77–84. https://doi.org/10.1080/03009734.2020.1728444
Boucret L, Tramon L, Saulnier P, Ferré-L’Hôtellier V, Bouet PE, May-Panloup P (2021) Change in the strategy of embryo selection with time-lapse system implementation-impact on clinical pregnancy rates. J Clin Med 10:4111. https://doi.org/10.3390/jcm10184111
Sainte-Rose R, Petit C, Dijols L, Frapsauce C, Guerif F (2021) Extended embryo culture is effective for patients of an advanced maternal age. Sci Rep 11:13499. https://doi.org/10.1038/s41598-021-92902-9
Ezoe K, Miki T, Akaike H, Shimazaki K, Takahashi T, Tanimura Y, Amagai A, Sawado A, Mogi M, Kaneko S, Ueno S, Coticchio G, Cimadomo D, Borini A, Rienzi L, Kato K (2023) Maternal age affects pronuclear and chromatin dynamics, morula compaction and cell polarity, and blastulation of human embryos. Hum Reprod 38:387–399. https://doi.org/10.1093/humrep/dead001
Kim HJ, Park JK, Eum JH, Song H, Lee WS, Lyu SW (2021) Embryo selection based on morphological parameters in a single vitrified-warmed blastocyst transfer cycle. Reprod Sci 28:1060–1068. https://doi.org/10.1007/s43032-020-00349-6
Van de Velde H, De Vos A, Joris H, Nagy ZP, Van Steirteghem AC (1998) Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod 13:3160–3164. https://doi.org/10.1093/humrep/13.11.3160
Park JK, Ahn SY, Seok SH, Park SY, Bang S, Eum JH, Kwak IP, Kim JW, Lee WS (2022) Clinical usability of embryo development using a combined qualitative and quantitative approach in a single vitrified-warmed blastocyst transfer: assessment of pre-vitrified blastocyst diameter and post-warmed blastocyst re-expansion speed. J Clin Med 11:7085. https://doi.org/10.3390/jcm11237085
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26:1270–1283. https://doi.org/10.1093/humrep/der037
ESHRE Working Group on Time-Lapse Technology, Apter S, Ebner T, Freour T, Guns Y, Kovacic B, Le Clef N, Marques M, Meseguer M, Montjean D, Sfontouris I (2020) Good practice recommendations for the use of time-lapse technology. Hum Reprod Open 2020:hoaa008. https://doi.org/10.1093/hropen/hoaa008
Kim MK, Park JK, Jeon Y, Choe SA, Lee HJ, Kim J, Chang EM, Kim JW, Lyu SW, Kim JY, Kwak IP, Lee WS, Yoon TK (2019) Correlation between morphologic grading and euploidy rates of blastocysts, and clinical outcomes in in vitro fertilization preimplantation genetic screening. J Korean Med Sci 34:e27. https://doi.org/10.3346/jkms.2019.34.e27
Hur C, Nanavaty V, Yao M, Desai N (2023) The presence of partial compaction patterns is associated with lower rates of blastocyst formation, sub-optimal morphokinetic parameters and poorer morphologic grade. Reprod Biol Endocrinol 21(1):12
Lensen S, Lantsberg D, Gardner DK, Sophian AD, Wandafiana N, Kamath MS (2022) The role of timing in frozen embryo transfer. Fertil Steril 118:832–838. https://doi.org/10.1016/j.fertnstert.2022.08.009
Venturas M, Shah JS, Yang X, Sanchez TH, Conway W, Sakkas D, Needleman DJ (2022) Metabolic state of human blastocysts measured by fluorescence lifetime imaging microscopy. Hum Reprod 37:411–427. https://doi.org/10.1093/humrep/deab283
Bebbere D, Coticchio G, Borini A, Ledda S (2022) Oocyte aging: looking beyond chromosome segregation errors. J Assist Reprod Genet 39:793–800. https://doi.org/10.1007/s10815-022-02441-z
Haas J, Meriano J, Bassil R, Barzilay E, Zilberberg E, Casper RF (2019) Developmental potential of slow-developing embryos: day-5 morulae compared with day-5 cavitating morulae. Fertil Steril 111:105–111. https://doi.org/10.1016/j.fertnstert.2018.08.053
Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, Campbell A (2021) Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update 27:848–865. https://doi.org/10.1093/humupd/dmab016
Coticchio G, Lagalla C, Sturmey R, Pennetta F, Borini A (2019) The enigmatic morula: mechanisms of development, cell fate determination, self-correction and implications for ART. Hum Reprod Update 25:422–438. https://doi.org/10.1093/humupd/dmz008
Ivec M, Kovacic B, Vlaisavljevic V (2011) Prediction of human blastocyst development from morulas with delayed and/or incomplete compaction. Fertil Steril 96:1473–1478.e2. https://doi.org/10.1016/j.fertnstert.2011.09.015
Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, Distratis V, Borini A (2017) Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod Biomed Online 34:137–146. https://doi.org/10.1016/j.rbmo.2016.11.008
Lagalla C, Coticchio G, Sciajno R, Tarozzi N, Zacà C, Borini A (2020) Alternative patterns of partial embryo compaction: prevalence, morphokinetic history and possible implications. Reprod Biomed Online 40:347–354. https://doi.org/10.1016/j.rbmo.2019.11.011
Tao J, Tamis R, Fink K, Williams B, Nelson-White T, Craig R (2002) The neglected morula/compact stage embryo transfer. Hum Reprod 17:1513–1518. https://doi.org/10.1093/humrep/17.6.1513
Yang H, Liu Y, Niu W, Yang Z, Wang Y, Jin H, Li G (2023) Correlation study of male semen parameters and embryo aneuploidy in preimplantation genetic testing for aneuploidy. Front Endocrinol (Lausanne) 13:1072176. https://doi.org/10.3389/fendo.2022.1072176
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM (2014) Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 29:1173–1181. https://doi.org/10.1093/humrep/deu033
Shi W, Zhou H, Chen L, Xue X, Shi J (2022) Live birth rate following frozen-thawed blastocyst transfer is higher in high-grade day 6 blastocysts than in low-grade day 5 blastocysts. Front Endocrinol (Lausanne) 13:1066757. https://doi.org/10.3389/fendo.2022.1066757
Ueno S, Uchiyama K, Kuroda T, Okimura T, Yabuuchi A, Kobayashi T, Kato K (2020) Establishment of day 7 blastocyst freezing criteria using blastocyst diameter for single vitrified-warmed blastocyst transfer from live birth outcomes: a single-center, large cohort, retrospectively matched study. J Assist Reprod Genet 37:2327–2335. https://doi.org/10.1007/s10815-020-01882-8
Hur C, Nanavaty V, Yao M, Desai N (2023) The presence of partial compaction patterns is associated with lower rates of blastocyst formation, sub-optimal morphokinetic parameters and poorer morphologic grade. Reprod Biol Endocrinol 21:12. https://doi.org/10.1186/s12958-023-01059-9
Venturas M, Yang X, Kumar K, Wells D, Racowsky C, Needleman DJ (2021) Metabolic imaging of human cumulus cells reveals associations among metabolic profiles of cumulus cells, patient clinical factors, and oocyte maturity. Fertil Steril 116:1651–1662. https://doi.org/10.1016/j.fertnstert.2021.07.1204
Richani D, Dunning KR, Thompson JG, Gilchrist RB (2021) Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update 27:27–47. https://doi.org/10.1093/humupd/dmaa043
Guo YH, Liu Y, Qi L, Song WY, Jin HX (2021) Can time-lapse incubation and monitoring be beneficial to assisted reproduction technology outcomes? A randomized controlled trial using day 3 double embryo transfer. Front Physiol 12:794601. https://doi.org/10.3389/fphys.2021.794601
Zhao J, Yan Y, Huang X, Sun L, Li Y (2019) Blastocoele expansion: an important parameter for predicting clinical success pregnancy after frozen-warmed blastocysts transfer. Reprod Biol Endocrinol 17:15. https://doi.org/10.1186/s12958-019-0454-2
Acknowledgements
The authors thank the other CHA Fertility Center Gangnam embryologists and physicians who contributed significantly to this project. This research was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI21C1560020021).
Funding
This work was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI21C1560020021).
Author information
Authors and Affiliations
Contributions
JW Kim and WS Lee: project development and manuscript writing/editing. JK Park: data collection and analysis, and manuscript writing/editing. YM Jeon, SY Bang, and IP Kwak: data collection and analysis, and manuscript writing. All authors contributed to revising the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures conducted in the study adhered to the ethical standards set by the Institutional Review Board of CHA Gangnam Medical Center (GCI 2023-07-010-001), following the 1964 Helsinki Declaration and its subsequent amendments, or equivalent ethical standards.
Consent to participate
The requirement of participant consent was waived owing to the study’s retrospective nature and the utilization of medical records.
Research involving human participants and/or animals
All procedures conducted in the study adhered to the ethical standards set by the institutional and national research committee, following the 1964 Helsinki Declaration and its subsequent amendments, or equivalent ethical standards.
Informed consent
The requirement of participant consent was waived owing to the study’s retrospective nature and the utilization of medical records.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
404_2024_7461_MOESM1_ESM.docx
Supplementary file1 Online Resource 1 Examples of morphologic compaction patterns. a Fully compacted morula; b partially compacted morula (DOCX 96 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, J.K., Jeon, Y., Bang, S. et al. Time-lapse imaging of morula compaction for selecting high‐quality blastocysts: a retrospective cohort study. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-024-07461-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00404-024-07461-x