Abstract
Liver steatosis is the most common chronic liver disease and affects 10–24% of the general population. As the grade of disease can range from fat infiltration to steatohepatitis and cirrhosis, an early diagnosis is needed to set the most appropriate therapy. Innovative noninvasive radiological techniques have been developed through MRI and US. MRI-PDFF is the reference standard, but it is not so widely diffused due to its cost. For this reason, ultrasound tools have been validated to study liver parenchyma. The qualitative assessment of the brightness of liver parenchyma has now been supported by quantitative values of attenuation and scattering to make the analysis objective and reproducible. We aim to demonstrate the reliability of quantitative ultrasound in assessing liver fat and to confirm the inter-operator reliability in different respiratory phases. We enrolled 45 patients examined during normal breathing at rest, peak inspiration, peak expiration, and semi-sitting position. The highest inter-operator agreement in both attenuation and scattering parameters was achieved at peak inspiration and peak expiration, followed by semi-sitting position. In conclusion, this technology also allows to monitor uncompliant patients, as it grants high reliability and reproducibility in different body position and respiratory phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common chronic liver disease is liver steatosis or fatty liver, which affects 10–24% of the general population [1]. Non-alcoholic fatty liver disease (NAFLD) is a chronic disease related not only to alcohol consumption but also to diabetes, hyperlipidemia, toxins, drugs, or genetic diseases [2, 3].
NAFLD is diffusing in the general population in two steps, the first step consists of metabolic syndrome, where insulin resistance induces liver parenchyma to stock fat developing liver steatosis [4, 5].
The second step provides the progression of liver steatosis and steatohepatitis (NASH), characterized by inflammation and chronic damage that may evolve into liver fibrosis and end-stage liver disease [6]. Late or delayed diagnosis without any lifestyle change may increase the risk of liver fibrosis or cirrhosis in the general population with consequent costs on the healthcare system, but even in patients after liver transplantation [7,8,9,10].
Liver biopsy has the limit of being an invasive procedure and it allows the examination of only a selected parenchyma, but it has been abandoned in clinical practice [11,12,13].
To overcome those limits innovative noninvasive radiological techniques have been developed through MRI and US [14,15,16,17].
Magnetic resonance imaging proton density fat fraction (MRI-PDFF) enables accurate, repeatable, and reproducible quantitative assessment of liver fat over the entire liver parenchyma, achieving high accuracy and sensitivity [18]. The diagnostic power of MRI-PDFF allows the detection even of the 5% of microscopic fat, so it has a higher sensitivity to detect early, but fundamental changes in liver fat content than liver biopsies [19, 20].
The major concern about the wide diffusion of MRI-PDFF is represented by its costs, limited diffusion, and patient compliance [21,22,23]. Besides standard protocols, radiomics has already been proposed to be a useful tool in the management of several pathologies [24,25,26].
More in detail, most recent studies are also proposing machine learning-based models to analyze liver parenchyma, but there are still few studies validated in clinical practice [15, 27, 28].
Considering the huge diffusion and reproducibility of the liver US, ultrasound software has been enriched by tools dedicated to hepatic fat quantification [29].
Despite it is well known that a high percentage of fat determines the brightness of liver parenchyma on US images, we have to underline that this is related to the scatter and to the attenuation of the ultrasound wave due to the amount of fats [30, 31].
In particular, B-Mode ultrasound allows to assess the grade of liver steatosis through: the evaluation of echogenicity of the liver compared to the renal cortex. Furthermore, the right liver attenuation with diaphragm visualization and the visualization of intra-hepatic vessels are commonly used in clinical practice [32].
The qualitative assessment of the morphology or the brightness of liver parenchyma has now been supported by quantitative values obtained from tissue microstructure characterization [33] through quantitative ultrasound (QUS) techniques.
There is only a few evidence about the inter-operator reliability of fat quantification tools in clinical practice [34]. There is also a lack of evidence of its reliability in selected categories of patients, in different body positions and respiratory phases [35].
So this study aims to demonstrate the reliability of quantitative ultrasound (QUS) in assessing liver fat volume measurements, to confirm the inter-operator reliability in the respiratory phases, but even in different body positions, in order to follow up uncompliant patients.
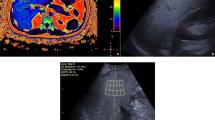
Tissue attenuation imaging (TAI, Samsung Medison)
Tissue attenuation is due to the energy loss of an ultrasound wave when it passes through a tissue. Attenuation depends on tissue features and wave frequency. When the percentage of liver fat is higher also the attenuation increases [36].
Attenuation coefficient (AC) has been calculated with several methods proposed by different vendors [37,38,39]. AC showed high reliability to detect liver fat, and to estimate the grade of liver steatosis, compared to liver biopsy and MRI-PDFF as reference standard [40].
In our study, AC is calculated by a parameter, the tissue attenuation imaging (TAI, Samsung Medison), that indicates the slope of the ultrasound central frequency downshift along the depth.
Tissue scatter distribution imaging (TSI, Samsung Medison)
Backscattering refers to the ultrasound energy reflected from a tissue, and it is represented by echogenicity or brightness. In particular, liver brightness means the backscattering has increased. The scattering of ultrasound also creates images with speckle patterns. The different patterns have been described by a statistical distribution. In particular, the Nakagami distribution correlates backscattering to the percentage of liver fat. In our work, Nakagami distribution is calculated by a parameter, tissue scatter distribution imaging (TSI, Samsung Medison), which calculates the concentration and the distribution of the ultrasound scatterers [41, 42].
TSI has been validated through a comparison with liver biopsy and MRI-PDFF as reference standards.
Materials and methods
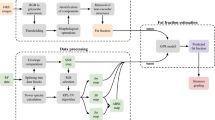
This is a retrospective study conducted on a prospectively collected database study conducted at the University of Molise between November 2022 and April 2023. The patients were admitted to an abdominal ultrasound examination for other reasons, at the University of Molise, Campobasso, Italy.
All patients signed an informed consent to publish their anonymous clinical data.
Inclusion criteria:
-
> 18 years old.
-
No history of chronic liver disease.
-
No habitual alcohol consumption.
Exclusion criteria:
-
< 18 years old.
-
Chronic liver disease or alcohol addiction.
-
Lack of compliance.
We studied echogenicity and composition of liver parenchyma.
For each patient beyond the US exam, we provided a dataset of clinical data: gender, age, body mass index (BMI), complete blood count, bilirubin and alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels.
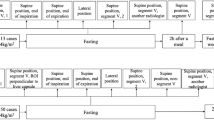
Patients were divided into three subgroups according to their body mass index: Subgroup 1 includes normal-weight patients with 18.5 < BMI < 24.99 kg/m2; subgroup 2 includes overweight patients with 25 < BMI < 29.99 kg/m2; and subgroup 3 includes obese patients with BMI > 30 kg/m2.
The ultrasound exam was performed on the right lobe with Samsung RS85 Prestige, with a single convex transducer 1–7 MHz convex transducer (CA1-7S) and completed with quantitative ultrasound (QUS) imaging: tissue attenuation imaging (TAI) and tissue scatter distribution imaging (TSI).
TAI was recorded only after verification of an “R2 value” < 0.8 [43].
The examinations were performed by 2 expert radiologists. A total of 10 measurements were recorded in different liver segments, in particular V, VI, VIII, and VII segments.
We included in the study the highest value of TAI and TSI found by each physician.
For each patient, a mean of 10 measurements was recorded using four different methods:
-
Method 1: normal breathing at rest.
-
Method 2: peak inspiration.
-
Method 3: end-expiration.
-
Method 4: semi-sitting position.
Patients were asked to inhale, hold their breath, exhale, or breathe quietly. Then, patients were asked to move to a semi-seating position.
Operators 1 and 2 conducted the US with the same machine settings.
Patient subgroups during examination are reported in Figs. 1, 2, and 3.
Standard of reference
Table 1 reported the reference standard selected following Sendur et al. [41]
Statistical analysis
Cohen's Kappa values were calculated to identify rates of inter-rater agreement between two different radiologists. Data were expressed as agreement in percentage, Cohen's Kappa value, standard error, and Z. The measure of the agreement below 0.0 means poor agreement, 0.00–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and > 0.80 almost perfect agreement [44, 45]. Considering that measurements were performed in different methods as normal breathing, peak inspiration, end-expiration, and semi-seating position, one-way ANOVA with Bonferroni correction was performed separately for both experts. Statistical significance was at p ≤ 0.05. Statistical analyses were performed with STATA SE 16.1 StataCorp LLC software.
Results
We retrospectively collected 45 patients and divided them into three subgroups: 15 normal-weight patients, 15 overweight patients, and 15 obese patients. The mean age of the patients was 36.7 ± 1.89 [24–65 CI].
Most of the patients were male (27/45, 60%). In particular, most of the obese patients were male (10/15, 75%).
The mean values of TAI and TSI recorded by the operators during normal breath were 0.718 ± 0.026 (operator 1), 0.755 ± 0.236 (operator 2) and 92,654 ± 1465 (operator 1) 92579 ± 2549 (operator 2). TAI and TSI values are both expressions of a population with a mean grade of steatosis 1. Inter-operators agreement in this phase was low, 15.56% for TAI and 2.22% for TSI.
During forced inspiration, the mean values of TAI and TSI recorded by the operators were 0.728 ± 0.023 (operator 1), 0.741 ± 0.0219 (operator 2) and 93.67 ± 1.809 (operator 1) 94.33 ± 1.84 (operator 2). Both the TAI and TSI values are coherent and belong to steatosis grade 1. In this respiratory phase, the inter-operator agreement was higher both for TAI and TSI measurement, 48.89% and 37.78%, respectively.
During peak expiration, the mean values of TAI and TSI recorded by the operators were 0.736 ± 0.024 (operator 1), 0.732 ± 0.029 (operator 2), and 92,658 ± 1605 (operator 1), 92,571 ± 1608 (operator 2). Also during this respiratory phase, TAI and TSI values are coherent and belong to steatosis grade 1. Inter-operator agreement was the highest achieved, both for TAI and TSI measurement, 48.89% and 48.89%, respectively.
In the semi-sitting position, in uncompliant patients, the mean values of TAI and TSI recorded by the operators were 0.701 ± 0.02 (operator 1), 0.715 ± 0.021 (operator 2), and 92,098 ± 1316 (operator 1), 94,277 ± 1457 (operator 2). Inter-operator agreement as medium–high, 33.33% both for TAI and TSI values.
Inter-operator agreement calculated with K-Cohen test showed the lowest K values in the measurement of TAI and TSI during quiet breath (K = 0.137 and K = 0.0115, respectively).
ANOVA test showed a significant statistical difference among operators only in the TSI measurements; therefore, quiet breath strongly influenced TSI value rather than TAI.
Results of statistical analysis are summarized in Tables 2 and 3.
Discussion
This study aimed to evaluate the impact on TAI and TSI values of the breathing cycle, chest movement, and body position, to validate the reliability of QUS. This validation allows operators to monitor even hospitalized or uncompliant patients in the most appropriate position or respiratory phase to overcome the limitations due to several artifacts. As for all new technologies the reliability and reproducibility of QUS are not completely tested.
After a statistical analysis, the ultrasound quantification of liver fat is confirmed to be reliable even in normal-weight patients, even in overweight patients, and even obese patients. The evaluation of attenuation and scattering achieved a high agreement among the operators, especially during the peak inspiration and peak expiration, but also a satisfying agreement in the semi-sitting position. The study conducted by Sendur et al. reported that inspiration and expiration do not significantly influence the results in patients with BMI > 25 kg/m2, while a significant difference in the attenuation coefficient in the BMI < 25 kg/m2 subgroup was found. Our study confirmed the reliability among the respiratory phases in overweight patients (BMI > 25 kg/m2), but also among the operators. In addition, there was not a significant difference in patients with BMI < 25 kg/m2 among the operators, among different respiratory phases. Also, in the BMI < 25 kg/m2 subgroup, there was a stronger agreement at peak inspiration and expiration, in spite of quiet breath. Concerning the different methods evaluated in our study, TAI measurement did not show any statistically significant difference among the respiratory phases, while TSI did, due to the higher variability in the quiet breath. This was also probably due to a higher sensitivity of the method to the thorax movement.
Affordability, portability, and wide availability are some of the many advantages of ultrasound in clinical practice, in comparison with other imaging techniques. Therefore, US tools could be efficiently used to diagnose and follow-up liver steatosis.
Comparing our data with the reference standard reported by Sendur et al., in our dataset there was neither underestimation nor overestimation of steatosis grade attributed to the patients among different methods [34, 46].
Anyway in this study, we focused on demonstrating the reliability of TAI and TSI measurements inter-operators in a stratified population composed of normal, overweight, and obese patients.
The main limitation of our study is the unavailability of an MRI-PDFF to compare the results. To overcome this limit we introduced a control group of normal-weight patients not affected by liver steatosis. TAI and TSI measurements, in fact, gradually increased from the normal-weight group to obese group.
The importance of detecting liver steatosis is already assessed as it affects 90% of obese patients [47].
Because the grade of injury can range from fat infiltration to cirrhosis, early therapy must be set [48, 49] and monitored to lose weight, rather than muscle mass [50,51,52].
Nowadays, the most effective treatments are represented by bariatric surgery in young patients [53], to avoid the development of metabolic syndrome and liver failure. It should be underlined, the high risk of liver steatosis also after liver transplants [54, 55], so QUS may represent a safe and efficient tool to monitor the results of bariatric surgery or the health of the liver graft.
Even if bariatric surgery might be a challenging surgical procedure and it might be considered an invasive treatment, especially for young people, the advent of minimally invasive surgery has changed surgical scenarios allowing faster recovery, lower blood loss, and lower risk of major complications [56,57,58,59,60,61,62,63,64].
Thanks to the low risk of complications, several studies are now introducing a combined treatment with both bariatric surgery before liver transplantation, as NAFLD is a metabolic condition that may persist in damaging the graft [65,66,67].
The combined treatment can be helpful in adult or elderly patients, even if a consensual physical performance is needed and further studies are needed to standardize the procedure [68, 69].
In these groups of patients who have undergone bariatric surgery or liver transplantation, the importance of follow-up to monitor liver fat is outstanding and there is the need to quantify the percentage of liver fat to avoid sub-optimal treatments [70].
In addition, as liver fibrosis can benefit weight loss, bariatric surgery is starting to be considered also in compensated patients [71].
Future investigation will focus on the implementation and validation of the shear wave parameter to evaluate and monitor liver fibrosis.
Future studies will concern the evaluation of attenuation and scattering in a prospective cohort of patients undergoing bariatric surgery and weight loss.
References
An L, Wirth U, Koch D et al (2022) The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. J Gastrointest Surg 26(3):671–683
Huang XJ, Yin M, Zhou BQ, Tan XY, Xia YQ, Qin CX (2023) Impact renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease in prevalence, characteristics and risk factors. World J Hepatol 15(8):985–1000. https://doi.org/10.4254/wjh.v15.i8.985
Song Y, Zhao F (2022) Bibliometric analysis of metabolic surgery for type 2 diabetes: current status and future prospects. Updates Surg 74(2):697–707. https://doi.org/10.1007/s13304-021-01201-5
Bellini MI, Urciuoli I, Del Gaudio G, Polti G, Iannetti G, Gangitano E, Lori E, Lubrano C, Cantisani V, Sorrenti S, D’Andrea V (2022) Nonalcoholic fatty liver disease and diabetes. World J Diabetes 13(9):668–682. https://doi.org/10.4239/wjd.v13.i9.668. (PMID: 36188142; PMCID: PMC9521438)
Faenza M, Benincasa G, Docimo L, Nicoletti GF, Napoli C (2022) Clinical epigenetics and restoring of metabolic health in severely obese patients undergoing batriatric and metabolic surgery. Updates Surg 74(2):431–438. https://doi.org/10.1007/s13304-021-01162-9
Csermely A, Mantovani A, Morieri ML et al (2023) Association between different modalities of insulin administration and metabolic dysfunction-associated fatty liver disease in adults with type 1 diabetes mellitus. Diabetes Metab. https://doi.org/10.1016/j.diabet.2023.101477
Ibrahim MK, Wilechansky RM, Challa PK et al (2023) The empirical dietary inflammatory pattern score and the risk of nonalcoholic fatty liver disease and cirrhosis. Hepatol Commun 7(10):e0263. https://doi.org/10.1097/HC9.0000000000000263
Fernandes ESM, de Mello FPT, Pimentel LS et al (2022) Combined liver transplantation with sleeve gastrectomy: a pioneer case series from Brazil. Updates Surg 74(1):81–86. https://doi.org/10.1007/s13304-021-01230-0
Fukushima M, Miyaaki H, Sasaki R et al (2023) Most cases of cryptogenic cirrhosis may be nonobese nonalcoholic steatohepatitis—risk factors of liver steatosis after liver transplantation for cryptogenic cirrhosis: a retrospective study. Intern Med 62(10):1415–1423. https://doi.org/10.2169/internalmedicine.0514-22
Lai Q, Ghinolfi D, Avolio AW, Manzia TM, Mennini G, Melandro F, Frongillo F, Pellicciaro M, Larghi Laureiro Z, Aglietti R, Franco A, Quaranta C, Tisone G, Agnes S, Rossi M, de Simone P (2022) Proposal and validation of a liver graft discard score for liver transplantation from deceased donors: a multicenter Italian study. Updates Surg 74(2):491–500. https://doi.org/10.1007/s13304-022-01262-0. (Epub 2022 Mar 11. PMID: 35275380; PMCID: PMC8995238)
Adani GL, Baccarani U, Sainz-Barriga M et al (2006) The role of hepatic biopsy to detect macrovacuolar steatosis during liver procurement. Transplant Proc 38(5):1404–1406. https://doi.org/10.1016/j.transproceed.2006.02.111
Choudhuri G, Shah S, Kulkarni A et al (2023) Non-alcoholic steatohepatitis in Asians: current perspectives and future directions. Cureus 15(8):e42852. https://doi.org/10.7759/cureus.42852
Renzulli M, Brandi N, Argalia G et al (2022) Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol Med 127(2):129–144. https://doi.org/10.1007/s11547-022-01449-w
Zalcman M, Barth RA, Rubesova E (2023) Real-time ultrasound-derived fat fraction in pediatric population: feasibility validation with MR-PDFF. Pediatr Radiol. https://doi.org/10.1007/s00247-023-05752-0
Qi YM, Xiao EH (2023) Advances in application of novel magnetic resonance imaging technologies in liver disease diagnosis. World J Gastroenterol 29(28):4384–4396. https://doi.org/10.3748/wjg.v29.i28.4384
Hyodo R, Takehara Y, Naganawa S (2022) 4D flow MRI in the portal venous system: imaging and analysis methods, and clinical applications. Radiol Med 127(11):1181–1198. https://doi.org/10.1007/s11547-022-01553-x
D’Onofrio M, de Sio I, Mirk P et al (2020) SIUMB recommendations for focal pancreatic lesions. J Ultrasound 23(4):599–606. https://doi.org/10.1007/s40477-020-00522-2
Dichtel LE, Tabari A, Mercaldo ND et al (2023) CT texture analysis in nonalcoholic fatty liver disease (NAFLD). J Clin Exp Hepatol 13(5):760–766. https://doi.org/10.1016/j.jceh.2023.04.001
Jang W, Song JS (2023) Non-invasive imaging methods to evaluate non-alcoholic fatty liver disease with fat quantification: a review. Diagnostics (Basel) 13(11):1852. https://doi.org/10.3390/diagnostics13111852
Gu J, Liu S, Du S et al (2019) Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 29(7):3564–3573. https://doi.org/10.1007/s00330-019-06072-4
Permutt Z, Le TA, Peterson MR et al (2012) Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 36(1):22–29. https://doi.org/10.1111/j.1365-2036.2012.05121
Huang DQ, Loomba R (2023) Editorial: will MRI-PDFF become the new standard for steatosis assessment in NAFLD? Aliment Pharmacol Ther 58(3):366–367. https://doi.org/10.1111/apt.17619
Faccioli N, Santi E, Foti G, D’Onofrio M (2022) Cost-effectiveness analysis of including contrast-enhanced ultrasound in management of pancreatic cystic neoplasms. Radiol Med 127(4):349–359. https://doi.org/10.1007/s11547-022-01459-8
Satake H, Ishigaki S, Ito R, Naganawa S (2022) Radiomics in breast MRI: current progress toward clinical application in the era of artificial intelligence. Radiol Med 127(1):39–56. https://doi.org/10.1007/s11547-021-01423-y
Rocca A, Brunese MC, Santone A et al (2021) Early diagnosis of liver metastases from colorectal cancer through CT radiomics and formal methods: a pilot study. J Clin Med 11(1):31. https://doi.org/10.3390/jcm11010031
Zerunian M, Pucciarelli F, Caruso D et al (2022) Artificial intelligence based image quality enhancement in liver MRI: a quantitative and qualitative evaluation. Radiol Med 127(10):1098–1105. https://doi.org/10.1007/s11547-022-01539-9
Sim KC, Kim MJ, Cho Y et al (2022) Radiomics analysis of magnetic resonance proton density fat fraction for the diagnosis of hepatic steatosis in patients with suspected non-alcoholic fatty liver disease. J Korean Med Sci 37(49):e339. https://doi.org/10.3346/jkms.2022.37.e339
Wang FH, Zheng HL, Li JT et al (2022) Prediction of recurrence-free survival and adjuvant therapy benefit in patients with gastrointestinal stromal tumors based on radiomics features. Radiol Med 127(10):1085–1097. https://doi.org/10.1007/s11547-022-01549-7
Kim BK, Bernstein N, Huang DQ et al (2023) Clinical and histologic factors associated with discordance between steatosis grade derived from histology vs. MRI-PDFF in NAFLD. Aliment Pharmacol Ther 58(2):229–237. https://doi.org/10.1111/apt.17564
Argalia G, Ventura C, Tosi N et al (2022) Comparison of point shear wave elastography and transient elastography in the evaluation of patients with NAFLD. Radiol Med 127(5):571–576. https://doi.org/10.1007/s11547-022-01475-8
Ferraioli G, Soares Monteiro LB (2019) Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol 25(40):6053–6062. https://doi.org/10.3748/wjg.v25.i40.6053)
Park J, Lee JM, Lee G, Jeon SK, Joo I (2022) Quantitative evaluation of hepatic steatosis using advanced imaging techniques: focusing on new quantitative ultrasound techniques. Korean J Radiol 23(1):13–29. https://doi.org/10.3348/kjr.2021.0112
Rónaszéki AD, Budai BK, Csongrády B et al (2022) Tissue attenuation imaging and tissue scatter imaging for quantitative ultrasound evaluation of hepatic steatosis. Medicine (Baltimore) 101(33):e29708. https://doi.org/10.1097/MD.0000000000029708
Zeng KY, Bao WY, Wang YH, Liao M, Yang J, Huang JY, Lu Q (2023) Non-invasive evaluation of liver steatosis with imaging modalities: new techniques and applications. World J Gastroenterol 29(17):2534–2550. https://doi.org/10.3748/wjg.v29.i17.2534. (PMID: 37213404; PMCID: PMC10198053)
Jeon SK, Lee JM, Joo I (2021) Clinical feasibility of quantitative ultrasound imaging for suspected hepatic steatosis: intra- and inter-examiner reliability and correlation with controlled attenuation parameter. Ultrasound Med Biol 47:438–445
Paige JS, Bernstein GS, Heba E et al (2017) A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol 208(5):W168–W177. https://doi.org/10.2214/AJR.16.16726
Bae JS, Lee DH, Lee JY et al (2019) Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol 29(12):6499–6507. https://doi.org/10.1007/s00330-019-06272-y
Fujiwara Y, Kuroda H, Abe T et al (2018) The B-mode image-guided ultrasound attenuation parameter accurately detects hepatic steatosis in chronic liver disease. Ultrasound Med Biol 44(11):2223–2232. https://doi.org/10.1016/j.ultrasmedbio.2018.06.017
Tamaki N, Koizumi Y, Hirooka M et al (2018) Novel quantitative assessment system of liver steatosis using a newly developed attenuation measurement method. Hepatol Res 48(10):821–828. https://doi.org/10.1111/hepr.13179
Bozic D, Podrug K, Mikolasevic I, Grgurevic I (2022) Ultrasound methods for the assessment of liver steatosis: a critical appraisal. Diagnostics (Basel) 12:2287
Şendur HN, Cerit MN, Ibrahimkhanli N, Şendur AB, Özhan Oktar S (2022) Interobserver variability in ultrasound-based liver fat quantification. J Ultrasound Med. https://doi.org/10.1002/jum.16048. (Epub ahead of print. PMID: 35778902)
Yun MH, Seo YS, Kang HS, Lee KG, Kim JH, An H, Yim HJ, Keum B, Jeen YT, Lee HS, Chun HJ, Um SH, Kim CD, Ryu HS (2011) The effect of the respiratory cycle on liver stiffness values as measured by transient elastography. J Viral Hepat 18(9):631–636. https://doi.org/10.1111/j.1365-2893.2010.01376.x. (Epub 2010 Oct 4 PMID: 21029256)
Ferraioli G, Kumar V, Ozturk A, Nam K, de Korte CL, Barr RG (2022) US attenuation for liver fat quantification: an AIUM-RSNA QIBA pulse-echo quantitative ultrasound initiative. Radiology 302(3):495–506. https://doi.org/10.1148/radiol.210736
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Şendur HN, Özdemir Kalkan D, Cerit MN, Kalkan G, Şendur AB, Özhan OS (2023) Hepatic fat quantification with novel ultrasound based techniques: a diagnostic performance study using magnetic resonance imaging proton density fat fraction as reference standard. Can Assoc Radiol J 74:362–369
Milić S, Lulić D, Štimac D (2014) Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol 20(28):9330–9337. https://doi.org/10.3748/wjg.v20.i28.9330
Voglino C, Badalucco S, Tirone A et al (2022) Follow-up after bariatric surgery: is it time to tailor it? Analysis of early predictive factors of 3-year weight loss predictors of unsuccess in bariatric patients. Updates Surg 74(4):1389–1398. https://doi.org/10.1007/s13304-022-01314-5
Agyapong G, Dashti F, Banini BA (2023) Nonalcoholic liver disease: epidemiology, risk factors, natural history, and management strategies. Ann N Y Acad Sci 1526(1):16–29. https://doi.org/10.1111/nyas.15012
Salaffi F, Carotti M, Di Matteo A et al (2022) Ultrasound and magnetic resonance imaging as diagnostic tools for sarcopenia in immune-mediated rheumatic diseases (IMRDs). Radiol Med 127(11):1277–1291. https://doi.org/10.1007/s11547-022-01560-y
Costa MSD, Pontes KSDS, Guedes MR, Barreto Silva MI, Klein MRST (2023) Association of habitual coffee consumption with obesity, sarcopenia, bone mineral density and cardiovascular risk factors: a two-year follow-up study in kidney transplant recipients. Clin Nutr 42(10):1889–1900. https://doi.org/10.1016/j.clnu.2023.08.004
Tagliafico AS, Bignotti B, Torri L, Rossi F (2022) Sarcopenia: how to measure, when and why. Radiol Med 127(3):228–237. https://doi.org/10.1007/s11547-022-01450-3
de Angelis F, Boru CE, Iossa A, Perotta N, Campanile FC, Silecchia G (2022) Long-term outcomes of sleeve gastrectomy as a revisional procedure after failed gastric band: a multicenter cross-matched cohort study. Updates Surg 74(2):709–713. https://doi.org/10.1007/s13304-021-01182-5
Elhelw O, Ragavan S, Majeed W et al (2023) The impact of bariatric surgery on liver enzymes in people with obesity: a 5-year observational study. Surgeon. https://doi.org/10.1016/j.surge.2023.07.006
Mak LY, Chan AC, Wong TC et al (2023) High prevalence of de novo metabolic dysfunction-associated fatty liver disease after liver transplantation and the role of controlled attenuation parameter. BMC Gastroenterol 23(1):307. https://doi.org/10.1186/s12876-023-02940-y
Thaher O, Driouch J, Hukauf M, Glatz T, Croner RS, Stroh C (2022) Is development in bariatric surgery in Germany compatible with international standards? A review of 16 years of data. Updates Surg 74(5):1571–1579. https://doi.org/10.1007/s13304-022-01349-8
Si Y, Lin S, Li C, Shen J, Guan W, Liang H (2023) Comparison of single versus double anastomosis bariatric metabolic surgery in obesity: a systematic review and meta-analysis. Obes Surg. https://doi.org/10.1007/s11695-023-06805-x
Polese L, Prevedello L, Belluzzi A, Giugliano E, Albanese A, Foletto M (2022) Endoscopic sleeve gastroplasty: results from a single surgical bariatric centre. Updates Surg 74(6):1971–1975. https://doi.org/10.1007/s13304-022-01385-4
Dupont L, Alves A, Bui E et al (2023) Long-term weight loss outcomes after bariatric surgery: a propensity score study among patients with psychiatric disorders. Surg Endosc. https://doi.org/10.1007/s00464-023-10343-z
Sakran N, Raziel A, Hod K, Azaria B, Goitein D, Kaplan U (2023) Early outcomes of one-anastomosis gastric bypass in the elderly population at high-volume bariatric centers. Updates Surg. https://doi.org/10.1007/s13304-023-01697-z. (Epub ahead of print. PMID: 38012431)
Kabir A, Izadi S, Mashayekhi F, Shokraee K, Rimaz S, Ansar H, Farsi F, Pazouki A (2023) Effect of different bariatric surgery methods on metabolic syndrome in patients with severe obesity. Updates Surg. https://doi.org/10.1007/s13304-023-01699-x. (Epub ahead of print. PMID: 38051454)
Ceccarelli G, Rocca A, De Rosa M et al (2021) Minimally invasive robotic-assisted combined colorectal and liver excision surgery: feasibility, safety and surgical technique in a pilot series. Updates Surg 73(3):1015–1022. https://doi.org/10.1007/s13304-021-01009-3
Ceccarelli G, Costa G, De Rosa M et al (2021) Minimally invasive approach to gastric GISTs: analysis of a multicenter robotic and laparoscopic experience with literature review. Cancers (Basel) 13(17):4351. https://doi.org/10.3390/cancers13174351
Ceccarelli G, Pasculli A, Bugiantella W et al (2020) Minimally invasive laparoscopic and robot-assisted emergency treatment of strangulated giant hiatal hernias: report of five cases and literature review. World J Emerg Surg 15(1):37. https://doi.org/10.1186/s13017-020-00316-1
de Barros F, Cardoso Faleiro Uba PH (2021) Liver transplantation and bariatric surgery: a new surgical reality: a systematic review of the best time for bariatric surgery. Updates Surg 73(5):1615–1622. https://doi.org/10.1007/s13304-021-01106-3
López-López V, Martínez-Serrano MÁ, Ruiz-Manzanera JJ, Eshmuminov D, Ramirez P (2023) Minimally invasive surgery and liver transplantation: is it a safe, feasible, and effective approach? Updates Surg 75(4):807–816. https://doi.org/10.1007/s13304-023-01506-7. (Epub 2023 Apr 4 PMID: 37014619)
Andrisani MC, Vespro V, Fusco S et al (2022) Interobserver variability in the evaluation of primary graft dysfunction after lung transplantation: impact of radiological training and analysis of discordant cases. Radiol Med 127(2):145–153. https://doi.org/10.1007/s11547-021-01438-5
Rocca A, Brunese MC, Cappuccio M et al (2021) Impact of physical activity on disability risk in elderly patients hospitalized for mild acute diverticulitis and diverticular bleeding undergone conservative management. Medicina (Kaunas) 57(4):360. https://doi.org/10.3390/medicina57040360
Komici K, Dello Iacono A, De Luca A, Perrotta F, Bencivenga L, Rengo G, Rocca A, Guerra G (2021) Adiponectin and sarcopenia: a systematic review with meta-analysis. Front Endocrinol (Lausanne) 15(12):576619. https://doi.org/10.3389/fendo.2021.576619. (PMID: 33935962; PMCID: PMC8082154)
Tavaglione F, De Vincentis A, Bruni V et al (2022) Accuracy of controlled attenuation parameter for assessing liver steatosis in individuals with morbid obesity before bariatric surgery. Liver Int 42(2):374–383. https://doi.org/10.1111/liv.15127
Miller A, Jalil S, Hussan H et al (2021) Apprising the choice of bariatric surgery in patients with compensated cirrhosis with morbid obesity: results of a national analysis. Updates Surg 73(5):1899–1908. https://doi.org/10.1007/s13304-021-01142-z
Acknowledgements
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Molise (Prot.n.19/2023).
Funding
Open access funding provided by Università degli Studi del Molise within the CRUI-CARE Agreement. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All patients enrolled signed the informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rocca, A., Komici, K., Brunese, M.C. et al. Quantitative ultrasound (QUS) in the evaluation of liver steatosis: data reliability in different respiratory phases and body positions. Radiol med 129, 549–557 (2024). https://doi.org/10.1007/s11547-024-01786-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-024-01786-y