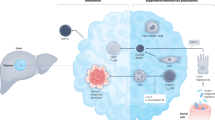
Abstract
Advanced intrahepatic cholangiocarcinoma (ICC) is a highly aggressive malignancy characterized by limited response to standard therapeutic modalities, such as radiotherapy, chemotherapy, and targeted therapy. The prognosis for patients with advanced ICC is exceedingly bleak, with an overall survival of less than 1 year. In recent years, personalized neoantigen vaccines have emerged as a promising approach to augment the immune response against tumors. Clinical investigations are currently underway to evaluate the efficacy of neoantigen-based peptide, DNA, and dendritic cell vaccines. Herein, we present a noteworthy case of advanced ICC patients who experienced disease progression following relapse and subsequently received immunotherapy with a personalized neoantigen nanovaccine. This innovative treatment strategy involved the administration of a custom-designed neoantigen-based peptide nanovaccine tailored to the patient’s specific gene mutation profile subsequent to failure of first-line therapy. The clinical efficacy and anti-tumor immune responses were evaluated using various methods, including imaging, interferon-γ ELISPOT assay, and intracellular cytokine staining. Notably, the neoantigen nanovaccine elicited a robust and specific tumor-killing effect mediated by T cells, resulting in a durable response lasting up to 25 months. These findings highlight the potential of neoantigen-based immunotherapy as a novel therapeutic avenue for the management of advanced ICC.


Similar content being viewed by others
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Brindley, P.J., M. Bachini, S.I. Ilyas, et al. 2021. Cholangiocarcinoma. Nature Reviews Disease Primers 7 (1): 65. https://doi.org/10.1038/s41572-021-00300-2.
Dodson, R.M., M.J. Weiss, D. Cosgrove, et al. 2013. Intrahepatic cholangiocarcinoma: management options and emerging therapies. Journal of the American College of Surgeons 217 (4): 736-750.e4. https://doi.org/10.1016/j.jamcollsurg.2013.05.021.
Jarnagin, W.R., Y. Fong, R.P. DeMatteo, et al. 2001. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Annals of Surgery 234 (4): 507–17. https://doi.org/10.1097/00000658-200110000-00010.
Hong, J.C., C.M. Jones, J.P. Duffy, et al. 2011. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Archives of Surgery 146 (6): 683–9. https://doi.org/10.1001/archsurg.2011.116.
Schumacher, T.N., and R.D. Schreiber. 2015. Neoantigens in cancer immunotherapy. Science 348 (6230): 69–74. https://doi.org/10.1126/science.aaa4971.
Sahin, U., E. Derhovanessian, M. Miller, et al. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547 (7662): 222–226. https://doi.org/10.1038/nature23003.
Ott, P.A., S. Hu-Lieskovan, B. Chmielowski, et al. 2020. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell 183 (2): 347-362.e24. https://doi.org/10.1016/j.cell.2020.08.053.
Wang, A.Z., R. Langer, and O.C. Farokhzad. 2012. Nanoparticle delivery of cancer drugs. Annual Review of Medicine 63: 185–98. https://doi.org/10.1146/annurev-med-040210-162544.
Shao, J., Q. Liu, J. Shen, et al. 2022. Advanced pancreatic cancer patient benefit from personalized neoantigen nanovaccine based immunotherapy: a case report. Fronties in Immunology 13: 799026. https://doi.org/10.3389/fimmu.2022.799026.
Weber, S.M., D. Ribero, E.M. O’Reilly, N. Kokudo, M. Miyazaki, and T.M. Pawlik. 2015. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 17 (8): 669–80. https://doi.org/10.1111/hpb.12441.
Hyder, O., I. Hatzaras, G.C. Sotiropoulos, et al. 2013. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 153 (6): 811–8. https://doi.org/10.1016/j.surg.2012.12.005.
Primrose, J.N., R.P. Fox, D.H. Palmer, et al. 2019. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. The Lancet Oncology 20 (5): 663–673. https://doi.org/10.1016/s1470-2045(18)30915-x.
Rizzo, A., and G. Brandi. 2021. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: reflections on a standard of care. Expert Review of Gastroenterolgy & Hepatology 15 (5): 483–485. https://doi.org/10.1080/17474124.2021.1864325.
Callahan, M.K., M.A. Postow, and J.D. Wolchok. 2016. Targeting T cell co-receptors for cancer therapy. Immunity 44 (5): 1069–78. https://doi.org/10.1016/j.immuni.2016.04.023.
Hoos, A. 2016. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nature Review Drug Discovery 15 (4): 235–47. https://doi.org/10.1038/nrd.2015.35.
Kwilas, A.R., R.N. Donahue, K.Y. Tsang, and J.W. Hodge. 2015. mmune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenvironment. https://doi.org/10.14800/ccm.677.
Kato, Y., K. Tabata, T. Kimura, et al. 2019. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One 14 (2).
Taylor, M.H., C.H. Lee, V. Makker, et al. 2020. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. Journal of Clinical Oncology 38 (11): 1154–1163. https://doi.org/10.1200/jco.19.01598.
Lidsky, M.E., Z. Wang, M. Lu, et al. 2022. Leveraging patient derived models of FGFR2 fusion positive intrahepatic cholangiocarcinoma to identify synergistic therapies. NPJ Precision Oncology 6 (1): 75. https://doi.org/10.1038/s41698-022-00320-5.
Hoy, S.M. 2020. Pemigatinib: first approval. Drugs 80 (9): 923–929. https://doi.org/10.1007/s40265-020-01330-y.
Rizzo, A., A.D. Ricci, G. Frega, A. Di Federico, and G. Brandi. 2021. FGFR inhibitors in elderly patients with advanced biliary tract cancer: an unsolved issue. Expert of Review Gastroenterology & Hepatology 15 (5): 567–574. https://doi.org/10.1080/17474124.2021.1911646.
Abou-Alfa, G.K., V. Sahai, A. Hollebecque, et al. 2020. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. The Lancet Oncology 21 (5): 671–684. https://doi.org/10.1016/s1470-2045(20)30109-1.
Lang, F., B. Schrörs, M. Löwer, Ö. Türeci, and U. Sahin. 2022. Identification of neoantigens for individualized therapeutic cancer vaccines. Nature reviews Drug discovery 21 (4): 261–282. https://doi.org/10.1038/s41573-021-00387-y.
Gubin, M.M., X. Zhang, H. Schuster, et al. 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515 (7528): 577–81. https://doi.org/10.1038/nature13988.
Acknowledgements
We thank the patient and her families, as well as the members who participated in the study, for making this research possible.
Funding
The study was supported by the National Natural Science Foundation of Nanjing University of Chinese Medicine (No. XZR2023075), the Hospital Management Research of Nanjing Drum Tower Hospital (No. NDYGN2023002), and Medical Research of Jiangsu Health Committee (No. H2023068).
Author information
Authors and Affiliations
Contributions
JShen and BRL designed the clinical trial. SHZ, CXL, JShen, and BRL drafted the manuscript and provided figures. SHZ, CXL, YCJ, HLZ, MZZ, CX, QL, JW, JShao, and JShen acquired, analyzed, and interpreted the data. JShen and BL revised the manuscript critically for important intellectual content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval
The patients/participants provided written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Liu, C., Jin, Y. et al. An Advanced Intrahepatic Cholangiocarcinoma Patient Benefits from Personalized Immunotherapy. Inflammation (2024). https://doi.org/10.1007/s10753-024-02003-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02003-8