Abstract
Background
Diagnosis of idiopathic pulmonary fibrosis (IPF) typically relies on high-resolution computed tomography imaging (HRCT) or histopathology, while monitoring disease severity is done via frequent pulmonary function testing (PFT). More reliable and convenient methods of diagnosing fibrotic interstitial lung disease (ILD) type and monitoring severity would allow for early identification and enhance current therapeutic interventions. This study tested the hypothesis that a machine learning (ML) ensemble analysis of comprehensive metabolic panel (CMP) and complete blood count (CBC) data can accurately distinguish IPF from connective tissue disease ILD (CTD-ILD) and predict disease severity as seen with PFT.
Methods
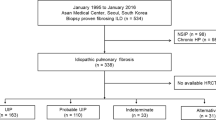
Outpatient data with diagnosis of IPF or CTD-ILD (n = 103 visits by 53 patients) were analyzed via ML methodology to evaluate (1) IPF vs CTD-ILD diagnosis; (2) %predicted Diffusing Capacity of Lung for Carbon Monoxide (DLCO) moderate or mild vs severe; (3) %predicted Forced Vital Capacity (FVC) moderate or mild vs severe; and (4) %predicted FVC mild vs moderate or severe.
Results
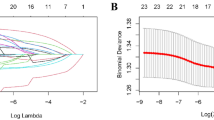
ML methodology identified IPF from CTD-ILD with AUCTEST = 0.893, while PFT was classified as DLCO moderate or mild vs severe with AUCTEST = 0.749, FVC moderate or mild vs severe with AUCTEST = 0.741, and FVC mild vs moderate or severe with AUCTEST = 0.739. Key features included albumin, alanine transaminase, %lymphocytes, hemoglobin, %eosinophils, white blood cell count, %monocytes, and %neutrophils.
Conclusion
Analysis of CMP and CBC data via proposed ML methodology offers the potential to distinguish IPF from CTD-ILD and predict severity on associated PFT with accuracy that meets or exceeds current clinical practice.



Similar content being viewed by others
Data availability
Data will be made available upon reasonable request.
References
U.S. National Library of Medicine, N.I.H. Idiopathic pulmonary fibrosis. https://ghr.nlm.nih.gov/condition/idiopathic-pulmonary-fibrosis. Accessed 30 Aug 2019
Raghu G et al (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183(6):788–824
Blackwell TS et al (2014) Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189(2):214–222
Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 379(8):797–798
Chung JH et al (2021) Differentiation of idiopathic pulmonary fibrosis from connective tissue disease-related interstitial lung disease using quantitative imaging. J Clin Med 10:12
Suzuki A, Kondoh Y, Fischer A (2017) Recent advances in connective tissue disease related interstitial lung disease. Expert Rev Respir Med 11(7):591–603
Erre GL et al (2021) Antifibrotic drugs in connective tissue disease-related interstitial lung disease (CTD-ILD): from mechanistic insights to therapeutic applications. Drugs Context 10:1
Hayton C et al (2019) Breath biomarkers in idiopathic pulmonary fibrosis: a systematic review. Respir Res 20:1
Hochhegger B et al (2019) Imaging in idiopathic pulmonary fibrosis: diagnosis and mimics. Clinics (Sao Paulo) 74:e225
Raghu G et al (2018) Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198(5):e44–e68
Guiot J et al (2017) Blood biomarkers in idiopathic pulmonary fibrosis. Lung 195(3):273–280
Hachisu Y et al (2019) Possible serological markers to predict mortality in acute exacerbation of idiopathic pulmonary fibrosis. Medicina (Kaunas) 55:5
Maher TM et al (2017) An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med 5(12):946–955
Zhang T et al (2021) KL-6 as an immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: a systematic review and meta-analysis. Front Immunol 12:745233
Zinellu A et al (2020) Blood cell count derived inflammation indexes in patients with idiopathic pulmonary fibrosis. Lung 198(5):821–827
Wuyts WA et al (2020) Idiopathic pulmonary fibrosis: best practice in monitoring and managing a relentless fibrotic disease. Respiration 99(1):73–82
Fernández Fabrellas E et al (2018) Prognosis and follow-up of idiopathic pulmonary fibrosis. Med Sci 6(2):51
Stephan S et al (2007) Oxygen desaturation during a 4-minute step test: predicting survival in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 24(1):70–76
Ley B, Collard HR, King TE Jr (2011) Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183(4):431–440
Nishiyama O et al (2016) Prognostic value of forced expiratory volume in 1 second/forced vital capacity in idiopathic pulmonary fibrosis. Chron Respir Dis 13(1):40–47
Ley B et al (2016) Predictors of mortality poorly predict common measures of disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 194(6):711–718
Travis WD et al (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188(6):733–748
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4:287–291
Nguyen L-P, Harper RW, Louie S (2016) Using and interpreting carbon monoxide diffusing capacity (DLco) correctly. Consultant 56(5):440–445
Dempsey TM, Scanlon PD (2018) Pulmonary function tests for the generalist: a brief review. In: Mayo Clinic proceedings. Elsevier, Amsterdam
Johnson JD, Theurer WM (2014) A stepwise approach to the interpretation of pulmonary function tests. Am Fam Phys 89(5):359–366
Raghu G et al (2022) Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 205(9):e18–e47
van Buuren S et al (2015) Package ‘mice’. Computer software
Waljee AK et al (2013) Comparison of imputation methods for missing laboratory data in medicine. BMJ Open 3(8):e002847
Jadhav A, Pramod D, Ramanathan K (2019) Comparison of performance of data imputation methods for numeric dataset. Appl Artif Intell 33(10):913–933
Stekhoven DJ, Bühlmann P (2012) MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 28(1):112–118
Collins GS et al (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med 13:1
Miller HA, van Berkel VH, Frieboes HB (2022) Lung cancer survival prediction and biomarker identification with an ensemble machine learning analysis of tumor core biopsy metabolomic data. Metabolomics 18(8):1–12
Grandini M, Bagli E, Visani G (2020) Metrics for multi-class classification: an overview. arXiv preprint arXiv:2008.05756
Fatourechi M et al (2008) Comparison of evaluation metrics in classification applications with imbalanced datasets. In: 2008 seventh international conference on machine learning and applications, 2008. IEEE
Katzenstein AL, Myers JL (2001) Idiopathic pulmonary fibrosis: to biopsy or not to biopsy. Am J Respir Crit Care Med 164(2):185–186
Hunninghake GW et al (2001) Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 164(2):193–196
Montesi SB et al (2020) Update in interstitial lung disease 2019. Am J Respir Crit Care Med 1:1
Vij R, Noth I (2012) Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res 159(4):218–227
Stainer A et al (2021) Molecular biomarkers in idiopathic pulmonary fibrosis: state of the art and future directions. Int J Mol Sci 22(12):6255
Krauss E et al (2019) Exploring the ability of electronic nose technology to recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): a pilot study from the European IPF Registry (eurIPFreg) and Biobank. J Clin Med 8(10):1698
Dragonieri S et al (2020) Exhaled volatile organic compounds analysis by e-nose can detect idiopathic pulmonary fibrosis. J Breath Res 14(4):047101
Hayton C et al (2019) Breath biomarkers in idiopathic pulmonary fibrosis: a systematic review. Respir Res 20(1):7
Yamada YI et al (2017) Volatile organic compounds in exhaled breath of idiopathic pulmonary fibrosis for discrimination from healthy subjects. Lung 195(2):247–254
Plantier L et al (2022) The use of exhaled air analysis in discriminating interstitial lung diseases: a pilot study. Respir Res 23(1):12
Taylor MJ et al (2024) Disease diagnosis and severity classification in pulmonary fibrosis using carbonyl volatile organic compounds in exhaled breath. Respir Med (in press)
Spagnolo P et al (2021) Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol Ther 222:107798
Cabrera Cesar E et al (2021) Serum biomarkers in differential diagnosis of idiopathic pulmonary fibrosis and connective tissue disease-associated interstitial lung disease. J Clin Med 10(14):3167
Raghu G et al (1999) The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest 116(5):1168–1174
Kishaba T et al (2021) Radiological and physiological predictors of IPF mortality. Medicina 57(10):1121
Zappala C et al (2010) Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 35(4):830–836
Hunninghake GW et al (1981) Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest 68(1):259–269
Schmid U et al (2021) Exposure-safety analyses of nintedanib in patients with chronic fibrosing interstitial lung disease. BMC Pulm Med 21(1):244
Lancaster L et al (2017) Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: experience from 92 sites in an open-label US expanded access program. Pulm Ther 3(2):317–325
Ikeda S et al (2017) Hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis: a single-center experience. Respir Investig 55(1):51–54
Soeters PB, Wolfe RR, Shenkin A (2019) Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr 43(2):181–193
Heinrich PC, Castell JV, Andus T (1990) Interleukin-6 and the acute phase response. Biochem J 265(3):621–636
Zisman DA et al (2009) Serum albumin concentration and waiting list mortality in idiopathic interstitial pneumonia. Chest 135(4):929–935
American Thoracic S, European Respiratory S (2002) American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165(2):277–304.
Zoz DF, Lawson WE, Blackwell TS (2011) Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci 341(6):435–438
Chen YH et al (2023) Clinical outcomes and risk factors of progressive pulmonary fibrosis in primary Sjogren’s syndrome-associated interstitial lung disease. BMC Pulm Med 23(1):268
Nathan SD et al (2020) The association between white blood cell count and outcomes in patients with idiopathic pulmonary fibrosis. Respir Med 170:106068
Xu L et al (2019) High hemoglobin is associated with increased in-hospital death in patients with chronic obstructive pulmonary disease and chronic kidney disease: a retrospective multicenter population-based study. BMC Pulm Med 19(1):174
Ruta VM et al (2020) Neutrophil-to-lymphocyte ratio and systemic immune-inflammation index-biomarkers in interstitial lung disease. Medicina (Kaunas) 56:8
Acknowledgements
Authors acknowledge partial funding by University of Louisville office of Executive Vice-President for Research and Innovation (EVPRI).
Author information
Authors and Affiliations
Contributions
Clinical study design: SS; Data collection: MT and SS; Machine learning implementation: AM; Machine learning analysis: AM, HM, and HF; Manuscript writing: AM and HF; Manuscript revision and editing: AM, HM, SS, and HF.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mueller, A.N., Miller, H.A., Taylor, M.J. et al. Identification of Idiopathic Pulmonary Fibrosis and Prediction of Disease Severity via Machine Learning Analysis of Comprehensive Metabolic Panel and Complete Blood Count Data. Lung 202, 139–150 (2024). https://doi.org/10.1007/s00408-024-00673-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-024-00673-7