Abstract
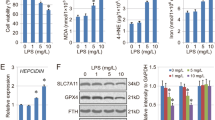
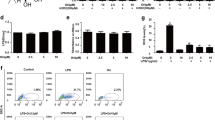
Acute lung injury (ALI) is a life-threatening clinical disorder with high mortality rate. Ferroptosis is a new type of programmed cell death with lipid peroxidation and iron ion overloading as the main characteristics. Endoplasmic reticulum (ER) stress and ferroptosis play pivotal roles in the pathogenesis of ALI. The study aimed to investigate the underlying relationship between ER stress and ferroptosis in ALI. The ER stress inhibitor 4-phenylbutyric acid (4-PBA) alleviated LPS-induced inflammation, and decreased IL-1β, IL-6, and TNF-α levels in BALF and lungs. The increased MDA and decreased GSH induced by LPS were partially reversed by 4-PBA, which also inhibited the expressions of ferroptosis-related protein ACSL4, COX-2, and FTH1. TEM further confirmed the ferroptosis within airway epithelia cells was ameliorated by 4-PBA. Moreover, 4-PBA reduced the production of ROS and lipid ROS in LPS-exposed BEAS-2B cells in a concentration-dependent way. Meanwhile, 4-PBA mitigated LPS-induced cell apoptosis in vivo and in vitro. Mechanistically, the MAPK signaling pathway activated by LPS was downregulated by 4-PBA. Collectively, these findings suggested that 4-PBA protected against ALI by inhibiting inflammation and ferroptosis through downregulating ER stress, thus providing a potential intervention for ALI and revealing the possible interaction between ER stress and ferroptosis in ALI.









Similar content being viewed by others
AVAILABILITY OF DATA AND MATERIALS
All supporting the findings of this report are included in this article. The data and materials are fully available upon reasonable request.
Abbreviations
- ALI:
-
Acute lung injury
- ER:
-
Endoplasmic reticulum
- 4-PBA:
-
4-Phenylbutyric acid
- HE:
-
Hematoxylin and eosin
- LPS:
-
Lipopolysaccharide
- ARDS:
-
Acute respiratory distress syndrome
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- BALF:
-
Bronchoalveolar lavage fluid
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- ACSL4:
-
Acyl-CoA synthetase long-chain family member 4
- COX-2:
-
Cyclooxygenase-2
- FTH1:
-
Ferritin heavy chain 1
- TEM:
-
Transmission electron microscopy
- ROS:
-
Reactive oxygen species
- IRE1α:
-
Inositol-requiring enzyme 1α
- PERK:
-
Protein kinase RNA-like endoplasmic reticulum kinase
- ATF6:
-
Activating transcription factor 6
- GRP78:
-
Glucose-regulated protein 78
- 4-HNE:
-
4-Hydroxynonenal
- ATF4:
-
Activating transcription factor 4
- TUNEL:
-
TdT-mediated dUTP nick end labeling
References
Thompson, B.T., R.C. Chambers, and K.D. Liu. 2017. Acute respiratory distress syndrome. The New England Journal of Medicine 377 (6): 562–572. https://doi.org/10.1056/NEJMra1608077.
Long, M.E., R.K. Mallampalli, and J.C. Horowitz. 2022. Pathogenesis of pneumonia and acute lung injury. Clinical Science (London, England: 1979) 136 (10): 747–769. https://doi.org/10.1042/CS20210879.
Acute respiratory distress syndrome. 2019. Nature Reviews Disease Primers 5 (1): 19. https://doi.org/10.1038/s41572-019-0075-2.
Yang, J.-X., M. Li, X.-O. Chen, Q.-Q. Lian, Q. Wang, F. Gao, et al. 2019. Lipoxin A ameliorates lipopolysaccharide-induced lung injury through stimulating epithelial proliferation, reducing epithelial cell apoptosis and inhibits epithelial-mesenchymal transition. Respiratory Research 20 (1): 192. https://doi.org/10.1186/s12931-019-1158-z.
Koppula, P., G. Lei, Y. Zhang, Y. Yan, C. Mao, L. Kondiparthi, et al. 2022. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nature Communications 13 (1): 2206. https://doi.org/10.1038/s41467-022-29905-1.
Pei, Z., Y. Qin, X. Fu, F. Yang, F. Huo, X. Liang, et al. 2022. Inhibition of ferroptosis and iron accumulation alleviates pulmonary fibrosis in a bleomycin model. Redox Biology 57: 102509. https://doi.org/10.1016/j.redox.2022.102509.
Bao, C., C. Liu, Q. Liu, L. Hua, J. Hu, Z. Li, et al. 2022. Liproxstatin-1 alleviates LPS/IL-13-induced bronchial epithelial cell injury and neutrophilic asthma in mice by inhibiting ferroptosis. International Immunopharmacology 109: 108770. https://doi.org/10.1016/j.intimp.2022.108770.
Li, J., K. Lu, F. Sun, S. Tan, X. Zhang, W. Sheng, et al. 2021. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. Journal of Translational Medicine 19 (1): 96. https://doi.org/10.1186/s12967-021-02745-1.
Yang, Y., Y. Ma, Q. Li, Y. Ling, Y. Zhou, K. Chu, et al. 2022. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death & Disease 13 (6): 530. https://doi.org/10.1038/s41419-022-04971-x.
Liu, P., Y. Feng, H. Li, X. Chen, G. Wang, S. Xu, et al. 2020. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cellular & Molecular Biology Letters 25: 10. https://doi.org/10.1186/s11658-020-00205-0.
Zhang, Y., L. Zheng, H. Deng, D. Feng, S. Hu, L. Zhu, et al. 2022. Electroacupuncture alleviates LPS-induced ARDS through α7 nicotinic acetylcholine receptor-mediated inhibition of ferroptosis. Frontiers In Immunology 13: 832432. https://doi.org/10.3389/fimmu.2022.832432.
Burman, A., H. Tanjore, and T.S. Blackwell. 2018. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biology 68–69: 355–365. Epub 2018/03/24. https://doi.org/10.1016/j.matbio.2018.03.015.
Bradley, K.L., C.A. Stokes, S.J. Marciniak, L.C. Parker, and A.M. Condliffe. 2021. Role of unfolded proteins in lung disease. Thorax 76 (1): 92–99. Epub 2020/10/21. https://doi.org/10.1136/thoraxjnl-2019-213738.
Borok, Z., M. Horie, P. Flodby, H. Wang, Y. Liu, S. Ganesh S, et al. 2020. Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. American Journal of Respiratory Critical Care Medicine 201 (2): 198–211. Epub 2019/11/19. https://doi.org/10.1164/rccm.201902-0451OC.
Delbrel, E., Y. Uzunhan, A. Soumare, T. Gille, D. Marchant, C. Planès, et al. 2019. ER stress is involved in epithelial-to-mesenchymal transition of alveolar epithelial cells exposed to a hypoxic microenvironment. International Journal of Molecular Sciences 20 (6). https://doi.org/10.3390/ijms20061299.
Pao, H.P., W.I. Liao, S.E. Tang, S.Y. Wu, K.L. Huang, and S.J. Chu. 2021. Suppression of endoplasmic reticulum stress by 4-PBA protects against hyperoxia-induced acute lung injury via up-regulating Claudin-4 expression. Frontiers in Immunology 12: 674316. Epub 2021/06/15. https://doi.org/10.3389/fimmu.2021.674316.
Zeng, M., W. Sang, S. Chen, R. Chen, H. Zhang, F. Xue, et al. 2017. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicology Letters 271: 26–37. https://doi.org/10.1016/j.toxlet.2017.02.023.
Dixon, S.J., K.M. Lemberg, M.R. Lamprecht, R. Skouta, E.M. Zaitsev, C.E. Gleason, et al. 2012. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149 (5): 1060–1072. https://doi.org/10.1016/j.cell.2012.03.042.
Yin, X., G. Zhu, Q. Wang, Y.D. Fu, J. Wang, and B. Xu. 2021. Ferroptosis, a new insight into acute lung injury. Frontiers In Pharmacology 12: 709538. https://doi.org/10.3389/fphar.2021.709538.
Jiang, X., B.R. Stockwell, and M. Conrad. 2021. Ferroptosis: Mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology 22 (4): 266-82. https://doi.org/10.1038/s41580-020-00324-8.
Forcina, G.C., and S.J. Dixon. 2019. GPX4 at the Crossroads of lipid homeostasis and ferroptosis. Proteomics 19 (18): e1800311. https://doi.org/10.1002/pmic.201800311.
Xu, Y., X. Li, Y. Cheng, M. Yang, and R. Wang. 2020. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB Journal 34 (12): 16262–16275. Epub 2020/10/19. https://doi.org/10.1096/fj.202001758R.
Zhong, H., and H. Yin. 2015. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biology 4: 193–199. https://doi.org/10.1016/j.redox.2014.12.011.
Xie, Y., W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, et al. 2016. Ferroptosis: process and function. Cell Death and Differentiation 23 (3): 369–379. Epub 2016/01/23. https://doi.org/10.1038/cdd.2015.158.
Qiu, Y.-B., B.-B. Wan, G. Liu, Y.-X. Wu, D. Chen, M.-D. Lu, et al. 2020. Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis. Respiratory Research 21 (1): 232. https://doi.org/10.1186/s12931-020-01500-2.
Li, L., D. Wu, S. Deng, J. Li, F. Zhang, Y. Zou, et al. 2022. NVP-AUY922 alleviates radiation-induced lung injury via inhibition of autophagy-dependent ferroptosis. Cell Death Discovery 8 (1): 86. https://doi.org/10.1038/s41420-022-00887-9.
Xu, M., J. Tao, Y. Yang, S. Tan, H. Liu, J. Jiang, et al. 2020. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Discovery 11 (2): 86. Epub 2020/02/06. https://doi.org/10.1038/s41419-020-2299-1.
Li, W., W. Li, Y. Leng, Y. Xiong, and Z. Xia. 2020. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA and Cell Biology 39 (2): 210–225. Epub 2019/12/07. https://doi.org/10.1089/dna.2019.5097.
Zhang, Z., L. Zhang, L. Zhou, Y. Lei, Y. Zhang, and C. Huang. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biology 25: 101047. Epub 2018/11/25. https://doi.org/10.1016/j.redox.2018.11.005.
Chen, D., Z. Fan, M. Rauh, M. Buchfelder, I.Y. Eyupoglu, and N. Savaskan N. 2017. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 36 (40): 5593–5608. Epub 2017/05/30. https://doi.org/10.1038/onc.2017.146.
Zhang, Q., J. Li, H. Zhong, and Y. Xu. 2021. The mechanism of nicotinamide on reducing acute lung injury by inhibiting MAPK and NF-κB signal pathway. Molecular Medicine (Cambridge, Mass) 27 (1): 115. https://doi.org/10.1186/s10020-021-00376-2.
Huang, X., G. Kong, Y. Li, W. Zhu, H. Xu, X. Zhang, et al. 2016. Decitabine and 5-azacitidine both alleviate LPS induced ARDS through anti-inflammatory/antioxidant activity and protection of glycocalyx and inhibition of MAPK pathways in mice. Biomedicine & Pharmacotherapy 84: 447–453. https://doi.org/10.1016/j.biopha.2016.09.072.
Stockwell, B.R. 2022. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 185 (14): 2401–2421. https://doi.org/10.1016/j.cell.2022.06.003.
Yang, L., L.-M. Cao, X.-J. Zhang, and B. Chu. 2022. Targeting ferroptosis as a vulnerability in pulmonary diseases. Cell Death & Disease 13 (7): 649. https://doi.org/10.1038/s41419-022-05070-7.
Chen, R., W. Zhong, C. Shao, P. Liu, C. Wang, Z. Wang, et al. 2018. Docosahexaenoic acid inhibits monocrotaline-induced pulmonary hypertension via attenuating endoplasmic reticulum stress and inflammation. American Journal of Physiology Lung Cellular and Molecular Physiology 314 (2): L243–L255. https://doi.org/10.1152/ajplung.00046.2017.
Kim, S.R., H.J. Kim, D.I. Kim, K.B. Lee, H.J. Park, J.S. Jeong, et al. 2015. Blockade of interplay between IL-17A and endoplasmic reticulum stress attenuates LPS-induced lung injury. Theranostics 5 (12): 1343–1362. Epub 2015/10/31. https://doi.org/10.7150/thno.11685.
Huang, B., D. He, G. Chen, X. Ran, W. Guo, X. Kan, et al. 2018. α-Cyperone inhibits LPS-induced inflammation in BV-2 cells through activation of Akt/Nrf2/HO-1 and suppression of the NF-κB pathway. Food Function 9 (5): 2735–2743. Epub 2018/04/19. https://doi.org/10.1039/c8fo00057c.
Chen, H.G., H.Z. Han, Y. Li, Y.H. Yu, and K.L. Xie. 2020. Hydrogen alleviated organ injury and dysfunction in sepsis: The role of cross-talk between autophagy and endoplasmic reticulum stress: Experimental research. International Immunopharmacology 78: 106049. Epub 2019/12/13. https://doi.org/10.1016/j.intimp.2019.106049.
Lee, Y.-S., D.-H. Lee, H.A. Choudry, D.L. Bartlett, and Y.J. Lee. 2018. Ferroptosis-induced endoplasmic reticulum stress: Cross-talk between ferroptosis and apoptosis. Molecular Cancer Research 16 (7): 1073–1076. https://doi.org/10.1158/1541-7786.MCR-18-0055.
Bouchaoui, H., L. Mahoney-Sanchez, G. Garçon, O. Berdeaux, L.Y. Alleman, D. Devos, et al. 2023. ACSL4 and the lipoxygenases 15/15B are pivotal for ferroptosis induced by iron and PUFA dyshomeostasis in dopaminergic neurons. Free Radical Biology & Medicine 195: 145–157. https://doi.org/10.1016/j.freeradbiomed.2022.12.086.
Yang, W.S., R. SriRamaratnam, M.E. Welsch, K. Shimada, R. Skouta, V.S. Viswanathan, et al. 2014. Regulation of ferroptotic cancer cell death by GPX4. Cell 156 (1–2): 317–331. https://doi.org/10.1016/j.cell.2013.12.010.
Chu, B., N. Kon, D. Chen, T. Li, T. Liu, L. Jiang, et al. 2019. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nature Cell Biology 21 (5): 579–591. https://doi.org/10.1038/s41556-019-0305-6.
Zou, Y., H. Li, E.T. Graham, A.A. Deik, J.K. Eaton, W. Wang, et al. 2020. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nature Chemical Biology 16 (3): 302–309. https://doi.org/10.1038/s41589-020-0472-6.
Liang, D., Y. Feng, F. Zandkarimi, H. Wang, Z. Zhang, J. Kim, et al. 2023. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186 (13). https://doi.org/10.1016/j.cell.2023.05.003.
Hassannia, B., P. Vandenabeele, and Berghe T. Vanden. 2019. Targeting ferroptosis to iron out cancer. Cancer Cell 35 (6): 830–849. https://doi.org/10.1016/j.ccell.2019.04.002.
Liu, Q., J. Wu, X. Zhang, X. Wu, Y. Zhao, and J. Ren. 2021. Iron homeostasis and disorders revisited in the sepsis. Free Radical Biology and Medicine 165. https://doi.org/10.1016/j.freeradbiomed.2021.01.025.
Cuschieri, J., and R.V. Maier. 2005. Mitogen-activated protein kinase (MAPK). Critical Care Medicine 33 (12 Suppl): S417–S419. Epub 2005/12/13. https://doi.org/10.1097/01.ccm.0000191714.39495.a6.
Wang, X., C. Zhang, N. Zou, Q. Chen, C. Wang, X. Zhou, et al. 2022. Lipocalin-2 silencing suppresses inflammation and oxidative stress of acute respiratory distress syndrome by ferroptosis via inhibition of MAPK/ERK pathway in neonatal mice. Bioengineered 13 (1): 508–520. https://doi.org/10.1080/21655979.2021.2009970.
Yi, S., K. Chen, L. Zhang, W. Shi, Y. Zhang, S. Niu, et al. 2019. Endoplasmic reticulum Ssress Is involved in stress-induced hypothalamic neuronal injury in rats via the PERK-ATF4-CHOP and IRE1-ASK1-JNK Pathways. Frontiers in Cellular Neuroscience 13: 190. https://doi.org/10.3389/fncel.2019.00190.
Zhu, K., X. Zhu, S. Sun, W. Yang, S. Liu, Z. Tang, et al. 2021. Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Experimental Neurology 345: 113828. https://doi.org/10.1016/j.expneurol.2021.113828.
Funding
This study was funded by the National Natural Science Foundation of China ([81873420] and [82070075]) and National Key Research and Development Plan, Ministry of Science and Technology of China (2018YFC0309502).
Author information
Authors and Affiliations
Contributions
Sijiao Wang and Fan Xu conceptualized and designed the study, validated the data, analyzed and curated the data, performed the experiments, performed software-related analysis, and wrote and revised the manuscript. Lei Zhu and Lijuan Hu conceptualized the study, validated and curated the data, acquired funding, obtained resources, revised the manuscript, supervised the study, and performed visualization and administration of the project. Hanhan Liu, Yue Shen and Jun Zhang performed the experiments and wrote the original draft of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Animal Care Committee of Fudan University Zhongshan Hospital.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Xu, F., Liu, H. et al. Suppressing Endoplasmic Reticulum Stress Alleviates LPS-Induced Acute Lung Injury via Inhibiting Inflammation and Ferroptosis. Inflammation (2024). https://doi.org/10.1007/s10753-023-01962-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-023-01962-8